Abstract
The ram’s horn squid Spirula spirula is a unique deep-water marine organism whose life cycle remains enigmatic. Interpretations of its ecology and habitat preferences are currently based solely on dredging, on fishery data, stable isotope data and rare molecular genetic analyses of dead specimens. These methods form the basis to decipher phylogeographic questions of otherwise unobservable deep-sea animals such as S. spirula. Here, new morphological data from internal shells (specimens n = 408, analysed n = 260) are presented from 12 different populations over huge distances, from the Atlantic, Indian and the Pacific Oceans. A monospecific status is assumed for Spirula, with its species S. spirula. The dataset shows a highly variable shell morphology including size distribution within distinct populations. Populations from the Indian Ocean are larger than those from the Atlantic and the Pacific. Specimens from the northern Indian Ocean (Maldives, Sri Lanka, Thailand) are larger than specimens from the eastern Indian Ocean (Mauritius, Tanzania) and the south-eastern Indian Ocean (western Australia). Specimens from the eastern Atlantic (Canary Islands) are smaller than those of the western Atlantic (Brazil, Tobago). The Canary Islands yielded by far the smallest specimens, while the largest specimen comes from Thailand. Specimens from the locality at eastern Australia (south-west Pacific) have an intermediate size range. Morphologic and geographic data suggest a geographically induced size differentiation within S. spirula. Preliminary findings on conchs mirror the known (from soft parts) existence of two sexual dimorphs in Spirula. The next step would be to collect more material from other localities. A more detailed morphometric approach based on specimens from which the sexes are known is required to enable a detection of the presence of sexual dimorphism by morphometric analyses on internal shells of Spirula.
Similar content being viewed by others
Introduction
Knowledge on the distribution, ecology, habitat and life cycle of the extant deep-water ram’s horn squid Spirula spirula (Decabrachia, suborder Spirulina) is still poor. The morphology of this peculiar deep-sea cephalopod is reinvestigated here.
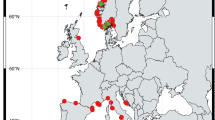
Spirula spirula occurs in open subtropical to tropical oceans from about 30°N to 30°S (d’Orbigny 1843; Clarke 1966, 1970, 1986; 10°N to 25°S in the Atlantic and 10°N to 35°S for Indian Ocean in Goud 1985; Nesis 1991; Okutani 1995; 50°N to 35°S in Haimovici et al. 2007; Lukeneder et al. 2008; Neige and Warnke 2010; Haring et al. 2012; Hoffmann and Warnke 2014; Fig. 1). Conclusions about its basic ecology and habitat preferences are mainly based on dredging and on fishery data (Chun 1910; Schmidt 1922; Kerr 1931; Bruun 1943; Clarke 1970; Haimovici et al. 2007). Clarke (1970) reported a life span for S. spirula of 18–20 months.
Distribution map of the extant deep-water squid S. spirula. Dark grey areas indicate live catches and thus the habitat of S. spirula. Light grey regions mark shells found drifting, washed ashore on beaches, and fishery bycatch. Dashed areas mark the possible, supposed or expected primary habitats, see text for explanation. Numbers 1–12 correspond to sites represented in the present comparative analysis. Atlantic Ocean: 1 Canary Islands, North Atlantic Ocean, South Spain, 2 Porto Santo, North Atlantic Ocean, South Portugal, 3 Tobago, Caribbean Sea, Trinidad & Tobago, 4 Salvador, East Brazil, South Atlantic Ocean, 5 Pater Noster Beach, South Atlantic Ocean, western South Africa. Indian Ocean: 6 var. localities, Indian Ocean, East Tanzania, 7 Mauritius, Indian Ocean, Republic of Mauritius, 8 var. localities Maldives, Laccadive Sea, Republic of the Maldives, 9 South-western Sri Lanka, Laccadive Sea, Republic of Sri Lanka, 10 Phuket, Andaman Sea, South Thailand. 11 Conspicuous Cliff & Sorrento Beach, South Indian Ocean, western Australia. Pacific Ocean: 12 Avalon Beach, Tasman Sea, eastern Australia. Distribution map compiled from literature and own data and modified after Schmidt (1922), Bruun (1943, 1955), Clarke (1966), Goud (1985), Nesis (1987, 1991), Joubin (1995), Reid (2005), Norman (2007), Lukeneder et al. (2008). For comparison see also Okutani (1995), Haimovici et al. (2007), Neige and Warnke (2010), Haring et al. (2012), and Hoffmann and Warnke (2014). Marine regions were classified in accordance with Claus et al. (2014a, b). For further details of specimen sites, see Table 1
Lukeneder et al. (2008) showed that stable isotopes (δ18O, δ13C) in the internal shells of dead S. spirula specimens can help decipher ontogenetic traits in this deep-sea squid. Well-preserved shells provide a reliable geochemical archive that reflects the life span migration cycle. Lukeneder et al. (2008), Price et al. (2009) and Warnke et al. (2010) used the aragonitic internal shells (Mutvei 1964; Dauphin 1976, 2001a, b; Watabe 1988; Doguzhaeva 1996, 2000; Doguzhaeva et al. 1999; Mutvei and Donovan 2006) as a geochemical proxy to interpret the ontogenetically related habitat changes. Subsequently, an ontogenetically controlled vertical migration was concluded.
After hatching in deep, cold seawater below 1000 m at temperatures around 4–6 °C, they start as a deep-water dweller during early ontogenetic stages but switch to warmer mid-water habitats (at 12–14 °C) during growth (Lukeneder et al. 2008; see Price et al. 2009, and Warnke et al. 2010). Finally, fully grown adults tend to retreat once again to slightly deeper and cooler environments. An identical threephase mode of life and vertical migration patterns for all specimens derived from the Atlantic and Pacific were documented. Beyond the insights into the ontogenetic vertical movement, nothing is known about the enigmatic squid’s lateral migration. Its patchy distribution range raises further questions about its phylogeography (Fig. 1). Over the last few years, new live catch data (Santos and Haimovici 2002; Perez et al. 2004; Haimovici et al. 2007; Neige and Warnke 2010; pers. comm. Deepika Suresh 2014) showed that the species distribution could be much wider than previously expected.
In the early publication history of the genus Spirula Lamarck 1799, numerous authors (Owen 1879; Huxley and Pelseneer 1895; Lönneberg 1896; see Chun 1910 and Lu 1998) noted up to six species within the genus: Spirula australis (see Chun 1910), S. blakei, S. fragilis, S. peroni (=S. peronii), S. prototypus, and S. recticulata (see Young and Sweeney 2002; WoRMS register, accessed 2015). From a genetic point of view, the Atlantic and Pacific populations should be genetically quite diverged. The aim of a recent study by Haring et al. (2012) was to reassess the postulate of species differentiation between the populations from the Atlantic and the Pacific (Warnke 2007) and to interpret the genetic data in a phylogeographic context (Warnke and Keupp 2005). In the three mitochondrial genes analysed, the distances between populations from the Atlantic and the Pacific are extremely low (Haring et al. 2012). Thus, molecular data suggest no species differentiation. So far only a few individuals of S. spirula have been investigated genetically. A recent and entire specimen caught near the southern Indian coast in 2014 by fishery will be analysed by DNA Barcoding (pers. comm. Deepika Suresh 2014). The expected molecular data will provide an additional component in the species composition of the genus.
Warnke (2007) postulated, concerning intrageneric variation, based on three individuals only, that the genus Spirula comprises two species, represented by the geographically widely separated Spirula populations from New Caledonia (South Pacific) and Fuerteventura (Canary Islands). Sequences from Fuerteventura (Warnke 2007) were compared with data from previously published sequences from New Caledonia (Bonnaud et al. 1996). Nesis (1998) challenged the monospecific status of Spirula due to its wide but patchy geographic (=oceanographic) range and the disconnection of South African and NW-African populations.
Recent data [DNA, three mitochondrial (mt) sequences] from Haring et al. (2012) on the cytochrome c oxidase subunit I (COI), the cytochrome oxidase subunit III (COIII), and presently available small ribosomal subunit rRNA (16S), compared to international data available in the GenBank, do not support the proposed split of Spirula into two species. The former data (Warnke 2007) are probably insufficient to claim species status for two distinct species. The low genetic distances observed over such a broad geographic range from the Pacific to the Atlantic are surprising. Haring et al. (2012) summarized that the genetic data (see also Bonnaud et al. 1994, 1996; Carlini and Graves 1999; Carlini et al. 2000; Lindgren et al. 2005; Warnke and Keupp 2005; Haring et al. 2012), even though scarce, suggest continuous genetic exchange among S. spirula populations and/or quite recent long distance dispersal. More data from deep-water live catches and additional molecular results are needed to support such interpretations. As Spirula inhabits deep-sea environments, targeted sampling is a rather hopeless task, but fishery can provide exceptional specimens. This makes it almost impossible to obtain the high numbers of specimens normally required for a comprehensive phylogeographic analysis (Haring et al. 2012). Each single specimen with soft tissue represents a crucial and important source of highly required information.
Based on morphometric analysis, Neige and Warnke (2010) assumed a poly-specific status in Spirula, emphasizing that more molecular investigations are needed. Their paper on morphological variations in geographically separated Spirula specimens states that morphological differences within different populations would indicate the existence of more than one species. Those authors further noted that certain shell parameters (e.g. size) vary with different geographic origins. As both Neige and Warnke (2010) and Haring et al. (2012) already noted, the morphology in Spirula cannot operate as the sole proxy for proving the species types within this group of cephalopods. The additional morphometric approach presented herein helps to understand these enigmatic deep-water squids.
Materials and methods
Shell material
The specimens of S. spirula analysed in the present study were found washed ashore on beaches after storm flooding or tidal activity (Fig. 2; Tables 1, 2). The pristine aragonitic shells, composed of three layers, of S. spirula derive from separate localities located in the Atlantic Ocean, the Indian Ocean and the Pacific Ocean (Figs. 1, 2; Tables 1, 2).
Side views of the aragonitic shells (maximum diameter specimens, only analysed specimen are figured) of S. spirula from the Atlantic Ocean, Indian Ocean and Pacific Ocean localities. a Canary Islands, NHMW 2015/0013/0001. b Tobago, NHMW 2015/0014/0001. c South Africa, NHMW 2015/0015/0001. d Brazil, 2008z0184/0008a. e Tanzania, NHMW 2015/0016/0001. f Mauritius, NHMW 2015/0017/0001. g Maldives, NHMW 2015/0018/0001. h Sri Lanka, NHMW 2015/0019/0001. i Thailand, 2007z0183/0015. j West Australia, encrusted by bryozoan colonies (Jellyella eburnea), NHMW 2015/0020/0001. k East Australia, encrusted by barnacles (Lepas pectinata), NHMW 2007z0171/0037. Scale bar for all specimens = 1 cm
A total of 408 shells of S. spirula were measured, and 260 were subsequently taken for detailed analysis (Table 3). Shell parameters [d, wh, ww, dm/wh, (wh/ww)/dm, and numerical size classes] of well-preserved shells show geographically separated populations with different ranges of diameters (mean and maximum values). The analysed shells range from 27.25 mm (specimen from Thailand) to 15.63 mm (Canary Islands) in maximum diameter and represented mostly fully grown adult specimens (see Neige and Warnke 2010). The phragmocone of the endogastrically coiled shells displayed 2.5 whorls segmented into 30 to 38 barrel-shaped chambers connected by a ventral siphuncle (see Lukeneder et al. 2008). The sex of the measured specimens is unknown.
Sampling sites
Examined specimens come from 12 sampling sites (Fig. 1; Tables 1, 2). Numbers of sampling sites are given according to their geographic position beginning with the Atlantic Ocean, followed by the Indian Ocean and finally one sampling site is located in the Pacific Ocean. Atlantic Ocean: site 1. Canary Islands, North Atlantic Ocean, southern Spain, site 2. Porto Santo, 40 km north-east of Madeira, North Atlantic Ocean, southern Portugal, site 3. Tobago, Caribbean Sea, Trinidad & Tobago, site 4. Salvador, eastern Brazil, South Atlantic Ocean, site 5. Pater Noster Beach, South Atlantic Ocean, western South Africa. Indian Ocean: site 6. var. localities, Indian Ocean, eastern Tanzania, site 7. Mauritius, 3 km north of Trou d’Eau Douce, Indian Ocean, Republic of Mauritius, site 8. var. localities Maldives, Laccadive Sea, Republic of the Maldives, site 9. South-west Sri Lanka, 1 km south of Bentoto and Hikkaduwa Beach, Laccadive Sea, Republic of Sri Lanka, site 10. Phuket, Andaman Sea, southern Thailand. site 11. Conspicuous Cliff, 12 km south-east of Walpole & Sorrento Beach, southern Indian Ocean, western Australia. Pacific Ocean: site 12. Avalon Beach, Tasman Sea, eastern Australia. For detailed coordinates of all sample sites see Table 2. Only a single and fragmented specimen was collected from site 2 (Porto Santo, Portugal), hence not included and analysed in Figs. 3, 4, 5, and 6.
a Bivariate plots of whorl height versus diameter of distinct populations. b Mean values and standard deviations are given for specimens of S. spirula from 11 geographic areas. Sample site numbers given in brackets correspond to site numbers of Fig. 1. The indicated coefficient of determination (R 2) is valid for the combined linear regression of all specimens measured
Diameter and whorl height in S. spirula from the Atlantic, Indian and Pacific Oceans. a Box plots showing geographic occurrences and diameter variations at distinct localities. b Box plots showing geographic occurrences and whorl height variation at distinct localities. Quartile method (rounded) in box plots
Numerical size classes (maximum diameter) in cumulation curves of S. spirula from the analysed material (complete specimens; n 260) of the Atlantic, Indian and Pacific Oceans. Sample site numbers given in brackets correspond to site numbers of Fig. 1
Variation of whorl sections by diameter versus whorl height/whorl width in S. spirula from the Atlantic, Indian and Pacific Oceans. a Bivariate plot showing size differences of distinct populations. b Mean values and standard deviations are given for whorl sections of S. spirula from 11 geographic areas. Sample site numbers given in brackets correspond to site numbers of Fig. 1. The indicated coefficient of determination (R 2) is valid for the combined linear regression of all specimens measured
The distribution map was compiled (data and maps) and modified after Schmidt (1922), Bruun (1943), Goud (1985), Lu et al. (1992), Reid and Norman (1998), Norman and Reid (2000), Reid (2005), Norman (2007), Lukeneder et al. (2008). For comparison see also Okutani (1995), Haimovici et al. (2007), Neige and Warnke (2010), Haring et al. (2012), Hoffmann and Warnke (2014), and own unpublished data. Marine regions were classified in accordance with Claus et al. (2014a, b).
Data collection and analyses
408 aragonitic shells, fragmented or complete, of S. spirula were measured; 260 complete or presumably adult specimens taken for detailed analysis. Maximum diameter (d), max. whorl height (wh) and max. whorl width (ww) were measured using a digital hand-held vernier calliper (SD 0.01 mm) and numerous smaller specimens additionally using a binocular microscope Zeiss Discovery.V20 combined with the digital camera Zeiss AxioCam MRc 5. The coiled shells displayed maximally 2.5 whorls, diameter was only measured if more than the last chambered half of the shell was preserved. Standard deviation (SD) is given for all data. Datasets were interpreted and graphically presented (box plots, mean, median, SD) using PAST (Hammer et al. 2001), Microsoft Excel 2010 and CorelDRAW X6. Maximum, minimum and mean values were calculated.
All samples are stored at the Natural History Museum of Vienna, in the collection of the Department of Geology and Palaeontology with inventory numbers from NHMW: 2007z0171/0001-0038, 2007z0183/0015, 2008z0184/0008a-d and 2009z0001/0001-0007, 2015/0013/0001-0032, 2015/0014/0001-0009, 2015/0015/0001-0009, 2015/0016/0001-0025, 2015/0017/0001-0040, 2015/0018/0001-0006, 2015/0019/0001-0004, and 2015/0020/0001-0003. Additional specimens are housed in the private collections of Ortwin Schultz and Peter Sziemer.
Results
Spirula spirula shells display different size distribution patterns, with different d max size classes, in all major oceans (e.g., Atlantic Ocean, Pacific Ocean and Indian Ocean; Figs. 2, 3, 4, 5, 6, 7). This trend is less pronounced in wh values and not detectable from ww values.
Measurements on S. spirula were performed from 11 different geographic sites (Fig. 1; Tables 2, 3, 4).
The sampling sites of S. spirula exhibited the following maximum, mean and minimum size values (i.e. diameters; Figs. 2, 3, 4, 6; Table 3).
Diameters
Populations from the Atlantic Ocean show a d max of 24.52 mm (South Africa), a d min of 15.63 mm (Canary Islands) and a d mean of 19.42 mm (SD 0.82). Populations from the Indian Ocean appear with a d max of 27.25 mm (Thailand), a d min of 17.26 mm (Tanzania and Thailand) and a d mean of 21.45 mm (SD 1.60). The population from the Pacific Ocean (eastern Australia) appears with a d max of 21.96 mm, a d min of 17.78 mm and a d mean of 20.00 mm (SD 0.99; Figs. 3, 4, 6; Table 2).
Whorl heights
Populations from the Atlantic Ocean show a whmax of 5.84 mm (Tobago), a whmin of 3.90 mm (Canary Islands) and a whmean of 5.35 mm (SD 0.19). Populations from the Indian Ocean appear with a whmax of 7.12 mm (Maldives), a whmin of 4.55 mm (Maldives) and a whmean of 5.54 (SD 0.38). The population from the Pacific Ocean (eastern Australia) appears with a whmax of 5.99 mm, a whmin of 4.58 mm and a whmean of 5.99 mm (SD 0.38; Figs. 3, 4, 6; Table 2).
Whorl widths
Populations from the Atlantic Ocean show a wwmax of 6.24 mm (Tobago), a wwmin of 4.39 mm (Canary Islands) and a wwmean of 5.34 mm (SD 0.19). Populations from the Indian Ocean appear with a wwmax of 7.44 mm (Maldives), a wwmin of 4.77 mm (Tanzania) and a wwmean of 5.86 mm (SD 0.39). The population from the Pacific Ocean (eastern Australia) appears with a wwmax of 6.59 mm, a wwmin of 5.03 mm and a wwmean of 5.66 mm (SD 0.42; Figs. 3, 4, 6; Table 3).
Whorl parameter indexes
Bivariate plots for conch height index (CHI, wh/d), conch width index (CWI; ww/d) and whorl width index (WWI, ww/wh) reveal that all measured S. spirula specimens from all major oceans (Atlantic Ocean, Pacific Ocean and Indian Ocean) are wider than high at final shell stages (maximal size = adult; Fig. 7; Table 4).
Populations from the Atlantic Ocean show a CHImax of 0.36 (Canary Islands), a CHImin of 0.22 (South Africa) and a CHImean of 0.28 (SD 0.02). Populations from the Indian Ocean appear with a CHImax of 0.33 (Tanzania), a CHImin of 0.22 (Maldives and Thailand) and a CHImean of 0.26 (SD 0.02). The population from the Pacific Ocean (eastern Australia) appears with a CHImax of 0.29, a CHImin of 0.24 and CHImean of 0.26 (SD 0.01; Fig. 7; Table 4).
Populations from the Atlantic Ocean show a CWImax of 0.38 (Canary Islands), a CWImin of 0.24 (South Africa) and a CWImean of 0.28 (SD 0.02). Populations from the Indian Ocean appear with a CWImax of 0.34 (Mauritius and Tanzania), a CWImin of 0.23 (Maldives and Thailand) and a CWImean of 0.28 (SD 0.02). The population from the Pacific Ocean (eastern Australia) appears with a CWImax of 0.32, a CWImin of 0.26 and CWImean of 0.28 (SD 0.01; Fig. 7; Table 4).
Populations from the Atlantic Ocean show a WWImax of 1.13 (Canary Islands), a WWImin of 1.02 (Canary Islands and Brazil) and a WWImean of 1.07 (SD 0.03). Populations from the Indian Ocean appear with a WWImax of 1.14 (Tanzania), a WWImin of 1.00 (Maldives) and a WWImean of 1.06 (SD 0.03). The population from the Pacific Ocean (eastern Australia) appears with a WWImax of 1.17, a WWImin of 1.04 and WWImean of 1.08 (SD 0.02; Fig. 7; Table 4).
These data indicate a connection between geography (i.e. geographic differences) and the absolute variation in adult animal size within a given population. As the shell size is a specific shell feature, geography alone does not affects a difference in adult shell size, The final shell size, measurable on the internal shells of this enigmatic deep-water squid, and growth mechanisms are rather being caused by oceanic water parameters as temperature, salinity, currents and food supply. According to Neige and Warnke (2010), the maximal shell size in Spirula as evidence for a fully grown, adult animal is indicated by the final decrease in wh of the shell (see Hoffmann and Reinhoff 2015). Variations in maximal size are detected not only between the different major oceans, but even within different intra-oceanic regions (e.g. Canary Islands/West Atlantic and Tobago/East Atlantic; Fig. 4).
The size records suggest 2 main size classes which correspond to 11 geographic sites. The maximum size (i.e. mean values) ranges from smaller forms with a minimum 15.63 mm (mean 17.39 mm, Canary Islands) to large-sized specimens with a maximum 27.25 mm (mean 22.82 mm, Thailand). A separation of the data into these postulated size classes or populations, plotted in d/wh, d/ww) or wh/ww bivariate plots and cross plots, revealed a clear separation of size field influenced by geographic occurrences (during lifetime) and distribution areas (plus post mortem drifting), comprising the separated clusters of varying adult size groups (=maximum size groups; Figs. 5, 6).
Maximum shell diameters, whorl heights and whorl width (i.e. adult size) apparently vary with geographic occurrence. Shells from the same geographic areas display similar growth patterns (i.e. absolute size, size distribution).
Specimens (max. size) from the Indian Ocean are larger than those from the Atlantic Ocean and the Pacific (Figs. 2, 3, 4, 5, 6, 7; Table 3). An exception is formed by the large specimens from the intermediate geographic/oceanographic position (south-eastern Atlantic vs. south-western Indian Oceans) from South Africa (i.e. Cape of Good Hope). In more detail, specimens from the northern Indian Ocean (Maldives, Sri Lanka, Thailand) are larger than specimens from the eastern Indian Ocean (Mauritius, Tanzania) and the south-eastern Indian Ocean (western Australia). Specimens from the eastern Atlantic (Canary Islands) are smaller than those of the western Atlantic (Brazil, Tobago). The Canary Islands yielded by far the smallest specimens and the Maldives the largest specimens from of all localities/oceans sampled (Fig. 4). Specimens of the locality at East Australia (south-western Pacific) show an intermediate size range around a mean of 20.0 mm. Similar and equivalent modifications and differences in size and growth can be observed in whorl height and whorl width values (Figs. 3, 4, 6; Table 3).
This confirms observations about differences in diameter, whorl height and whorl width in adult stages between the main geographic populations.
Discussion
Measurements on coiled extant (e.g. Spirula, Nautilus) and fossil cephalopods (e.g. ammonoids, nautilids) show variations of maximum size parameters in accordance with age, ecology and/or even geography (Reboulet 2001; Landman et al. 2003; Lukeneder 2004). During the last decade, numerous papers have investigated the deep-water squid S. spirula with regard to the challenging species enigma. It remains unresolved whether there is only a single species (monospecific; Chun 1910; see only 1 species in WoRMS register, accessed 2015) or several distinct species (poly-specific). On one hand, analyses of genetic material (Bonnaud et al. 1994; Carlini and Graves 1999; Carlini et al. 2000; Nishiguchi et al. 2004; Lindgren et al. 2005; Akasaki et al. 2006; Warnke 2007; Haring et al. 2012) and on the other hand morphometric analyses (Neige and Warnke 2010) were performed to tackle this issue. Haring et al. (2012) concluded that the molecular data do not suggest species differentiation and hence no split of Spirula into two species (see also WoRMS register, accessed 2015), as supposed by Warnke (2007). The proposed split by Warnke (2007) is therefore most probably based on artefacts.
The morphometric results presented herein can help understand these enigmatic deep-water squids. The distribution of size classes and maximum size ranges agree with data given in Neige and Warnke (2010). The morphometric analysis of Neige and Warnke (2010) pointed to a poly-specific status of Spirula, but emphasized the need for more molecular investigations. Their paper on morphological variations in geographically separated specimens states that morphological differences within different populations would indicate the existence of more than one species. The authors also noted that certain shell parameters (e.g. size) vary with different geographic origin. Neige and Warnke (2010) concluded based on their morphometric data on S. spirula that specimens from Madagascar, New Zealand and Brazil are larger than those from north-western Africa and Australia. Data presented herein confirm this conclusion and add more details to the datasets known so far. A highly variable spire morphology, size and style of whorls (coiling, expansion rate etc.) within distinct populations are observable.
Maximum size classes from catches and beach finding dates (January to December) are the same all over the year. No size dependence on geographic latitude, hence to water temperature variations and ranges, could be detected in the present analyses. The distance and the geographic position to the equator apparently have no direct influence to shell sizes. Note, however, that ocean surface temperatures are not the crucial parameters for the life of Spirula (Lukeneder et al. 2008; for oceanic temperatures see Mehra and Rivin 2010; GRTOFS 2015). As also shown by stable isotope studies (Lukeneder et al. 2008; Price et al. 2009, and Warnke et al. 2010) on S. spirula shells, the life cycle takes place within a water depth range from 1000 m up to 200 m (Bruun 1955; Clarke 1970; Reid 2005; Haimovici et al. 2007; Hoffmann and Warnke 2014). Reid (2005) and Warnke and Keupp (2005) concluded a distribution of S. spirula in subtropical and tropical oceanic waters where the water temperature is 10 °C or warmer at a depth of 400 m (Lu 1998; Reid 2005), 800 m (Hoffmann and Warnke 2014) and 1000 m (Warnke and Keupp 2005; for oceanic temperatures see Mehra and Rivin 2010; GRTOFS 2015). Isotope data, and hence the resulting water temperatures for the corresponding areas during ontogeny, are almost identical for all analysed Atlantic Ocean, Indian Ocean and Pacific Ocean specimens (Lukeneder et al. 2008). Size class ranges and maximum diameters within the distinct populations presented herein show no dependence or correspondence to any route following oceanic deep-sea currents such as the great ocean conveyor belt; none of the known deep ocean currents fit the observed “patchy” distribution of S. spirula.
Recent findings on the distribution of S. spirula favour an explanation involving the global distribution of a single species (Haring et al. 2012). Accordingly, an undetected (so far) huge panmictic population with continuous gene flow exists, what needs to be tested. Live catch data accompanied with reported drift shells washed on beaches (Bruun 1943; Clarke 1986; Goud 1985; Lukeneder et al. 2008; Norman 2007; Okutani 1995; Schmidt 1922; Reid 2005) yielded various different distribution maps. New data from Brazil gained by trawling and bycatch (Perez et al. 2004; Haimovici et al. 2007) and by investigating stomach contents of fishes and other squids (e.g. diverse teleosts and Illex argentinus; Santos and Haimovici 2002) showed that the distribution of this deep-water squid could be much wider than expected based on previous data. This might be explained by connections in mid- and deep-water areas around the world´s oceans. Nonetheless, even in this case, the low genetic distances shown in Haring et al. (2012) over such a broad geographic range are surprising (note dashed lines in Fig. 1).
A further interesting but complicated issue is the possible existence of sexual dimorphism, visible not only in soft bodies (see Chun 1910; Clarke 1970; Warnke 2007; Hoffmann and Warnke 2014) but notably also on hard parts (i.e. internal shell). The material from the Maldives (n = 70, with indicated coefficient of determination R 2, Fig. 8) was used to test for a visible difference in S. spirula shells. The subjective first impression of two distinct morphotypes within the Maldives population seems to be mirrored in the graphic bivariate plot of whorl height versus diameter. “Delicate” (smaller wh at same d) forms plot below a defined borderline, whereas more “robust” (larger wh at same d) plot above that line. This trend encompassed both juvenile (smaller) and adult (bigger) specimens (Fig. 8). The ww/d plot, as in wh/d, again shows a separation of 2 main morphogroups. The subjective delicate specimens plot below an imaginary dashed line (drawn after plotting). More robust specimens from morphotype 1 plot above the area of morphotype 2 specimens. This separation is not visible in the wh/ww versus d plot (Fig. 8c). Two distinct morphotypes of shells within the Maldives population seem to mirror sexual dimorphism, visible on the hardparts. The preliminary findings on the existence of two sexual dimorphs will benefit from more material from other localities. As noted by Neige and Warnke (2010), a morphometric approach based on specimens from which the sexes are known (soft parts with internal shells) would be required to control the presence of sexual dimorphism by morphometric analyses.
Bivariate diagrams of conch parameters of S. spirula from the Maldives with indicated coefficient of determination (R 2). R 2 values are valid for the combined linear regression of both morphotype groups. a Bivariate plot of whorl height (wh) versus diameter (d). b Bivariate plot of whorl width (ww) versus d. c Bivariate plot of wh/ww versus d. Note the dashed line between morphotype 1 (robust specimens, grey circles) and morphotype 2 (delicate specimens, black triangles; see text for explanation)
In summary, genetic data, even though scarce, suggest continuous genetic exchange among S. spirula populations and/or quite recent long distance dispersal, although the latter assumption still requires a conclusive explanation for the mediating mechanisms. More data from deep-water live catches and additional molecular results will help solidify these interpretations.
References
Akasaki, T., Nikaido, M., Tsuchiya, K., Segawa, S., Hasegawa, M., & Okada, N. (2006). Extensive mitochondrial gene arrangements in coleoid Cephalopoda and their phylogenetic implications. Molecular Phylogenetics and Evolution, 38, 648–658.
Bonnaud, L., Boucher-Rodoni, R., & Monnerot, M. (1994). Phylogeny of decapod cephalopods based on partial 16S rDNA nucleotide sequences. Comptes Rendus de l Academie des Sciences. Serie III, Sciences de la Vie (Paris), 317, 581–588.
Bonnaud, L., Boucher-Rodoni, R., & Monnerot, M. (1996). Relationships of some coleoid cephalopods established by 3′end of the 16S rDNA and cytochrome oxidase III gene sequence comparison. American Malacological Bulletin, 12, 87–90.
Bruun, A. F. (1943). The biology of Spirula spirula (L.). Dana Report, 24, 1–48.
Bruun, A. F. (1955). New light on the biology of Spirula, a mesopelagic cephalopod. Essays in the natural sciences in honour of Captain Allan Hancock (pp. 61–71). Los Angeles: University of California Press.
Carlini, D. B., & Graves, J. E. (1999). Phylogenetic analysis of cytochrome c oxidase I sequences to determine higher-level relationships within the coleoid cephalopod. Bulletin of Marine Science, 64, 57–76.
Carlini, D. B., Reece, K. S., & Graves, J. E. (2000). Actin gene family evolution and the phylogeny of coleoid cephalopods (Mollusca: Cephalopoda). Molecular Biology and Evolution, 17, 1353–1370.
Chun, C. (1910). Die Cephalopoden. 2 Teil: Myopsida. Wissenschaftliche Ergebnisse der Deutschen Tiefsee-Expedition auf dem Dampfer „Valdivia“ 1898–1899, 18, 403–476.
Clarke, M. R. (1966). A review of the systematics and ecology of oceanic squids. In F. S. Russel (Ed.), Advances in marine biology (Vol. 4, pp. 91–300). London: Academic Press.
Clarke, M. R. (1970). Growth and development of Spirula spirula. Journal of the Marine Biological Association of the United Kingdom, 50, 53–64.
Clarke, M. R. (1986). A handbook for the identification of cephalopod beaks (p. 273). Oxford: Clarendon Press.
Claus, S., De Hauwere, N., Vanhoorne, B., Deckers, P., Souza Dias, F., Hernandez, F., & Mees, J. (2014a). Marine regions: Towards a global standard for georeferenced marine names and boundaries. Marine Geodesy, 37(2), 99–125.
Claus, S., De Hauwere, N., Vanhoorne, B., Souza Dias, F., Hernandez, F., & Mees, J. (2014b). MarineRegions.org., Flanders Marine Institute. Retrieved November 26, 2014 from http://www.marineregions.org.
D’Orbigny, A. (1843). Cephalopods. In Gide et Cie (Eds.), Mollusques vivants et fossiles ou description des toutes les espéces de coquilles et de Mollusques, classées suivant leur distribution géologique et géographique (pp. 605, Tome 1, with atlas). Paris.
Dauphin, Y. (1976). Microstructure des coquilles de Céphalopodes. I. Spirula spirula L. (Dibranchiata, Decapoda). Bulletin du Museum National d’Histoire Naturelle de Paris (3èm sér.), 382, Sciences de la Terre, 54, 197–238.
Dauphin, Y. (2001a). Caractéristiques de la phase organique soluble des tests aragonitiques des trios genres de cephalopods actuels. Neues Jahrbuch für Geologie und Paläontologie Monatshefte, 2001(2), 103–123.
Dauphin, Y. (2001b). Nanostructures de la nacre des tests de cephalopods actuels. Paläontologische Zeitschrift, 75, 113–122.
de Lamarck, J. B. P. A. (1799). Prodrome d’une nouvelle classification des coquilles. Mémoires de la Société d’Histoire Naturelle de Paris, 1, 63–91.
Doguzhaeva, L. A. (1996). Two Early Cretaceous spirulid coleoids of the North-Western Caucasus: Their shell ultrastructure and evolutionary implications. Palaeontology, 39, 681–709.
Doguzhaeva, L. A. (2000). The evolutionary morphology of siphonal tube, in Spirulida (Cephalopoda, Coleoidea). Revue Paléobiologie, Volume Spécial, 8, 83–94.
Doguzhaeva, L. A., Mapes, R. H., & Mutvei, H. (1999). A late Carboniferous spirulid coleoid from the southern Mid-Continent (USA). In F. Oloriz & F. J. Rodriguez-Tovar (Eds.), Advancing research on living and fossil cephalopods (pp. 47–57). New York: Kluwer Academic/Plenum Publishers.
Goud, J. (1985). Spirula spirula, een inktvis uit de diepzee. In Vita Marina Zeebiologische Dokumentatie: zeebiologie, zeeaquariologie, malacologie (pp. 35–42), Stichting Biologia Maritima, Den Haag.
GRTOFS, Atlantic Real-Time Ocean Forecast System. (2015). A real time ocean forecast system for the North Atlantic Ocean. Retrieved November 17, 2014 from http://polar.ncep.noaa.gov/global/.
Haimovici, M., Costa, A. S., Santos, R. A., Martins, A. S., & Olavo, G. (2007). Composicao de species, distributicao e abundancia de cefalopodes do talude de regiao central Brasil. In A. S. Costa, G. Olavo, & A. S. Martins (Eds.), Biodiversidade da Fauna Mrinha Profunda na Costa central Brasileira (Vol. 24, pp. 109–132). Rio de Janeiro: Museu Nacional.
Hammer, Ø., Harper, D. A. T., & Ryan, P. D. (2001). Past: Paleontological statistics software package for education and data analysis. Palaeontologia Electronica, 4/1/4: pp. 9 178 kb. http://palaeo-electronica.org/2001_1/past/issue1_01.htm.
Haring, E., Kruckenhauser, L., & Lukeneder, A. (2012). New DNA sequence data on the enigmatic Spirula spirula (Linnaeus 1758) (Decabrachia, suborder Spirulina). Annalen des Naturhistorischen Museums Wien, 113B, 37–48.
Hoffmann, R., & Reinhoff, D. (2015). Non-invasive imaging techniques combined with morphometry: A case study from Spirula. In D. Fuchs, R. Hoffmann, & C. Klug. (Eds), Cephalopods—Present and past. Swiss Journal of Palaeontology. doi:10.1007/s13358-015-0083-0.
Hoffmann, R., & Warnke, K. (2014). Spirula—das unbekannte Wesen aus der Tiefsee. Denisia, 32, 33–46.
Huxley, T. H., & Pelseneer, P. (1895). Report on the specimen of the genus Spirula collected by H.M.S. Challenger. Report on the scientific results of the voyage of H.M.S. Challenger during the years 1873–76 under the command of Captain Georges S. Nares and the late Captain Frank Tourle Thomson. Appendix Zoology, 83, 1–32.
Joubin, L. (1995). Cephalopods from the scientific expeditions of Prince Albert I of Monaco. vol. I, parts 1–III; vol. 2 parts III–IV. Washington, DC: Smithsonian Institution Libraries and National Science Foundation.
Kerr, J. G. (1931). Notes upon the Dana specimens of Spirula and upon certain problems of cephalopod morphology. Dana report, 8, 1–34.
Landman, N. H., Klofak, S. M., & Sarg, K. B. (2003). Variation in adult size of scaphitid ammonites from the Upper Cretaceous Pierre Shale and Fox Hill Formation. In P. J. Harries (Ed.), Approaches in high-resolution stratigraphic paleontology (pp. 149–194). Dordrecht: Kluwer Academic Publishers.
Lindgren, A. R., Katugin, O. N., Amezquita, E., & Nishiguchi, M. K. (2005). Evolutionary relationships among squids of the family Gonatidae (Mollusca: Cephalopoda) inferred from three mitochondrial loci. Molecular Phylogenetics and Evolution, 36, 101–111.
Lönneberg, E. (1896). Notes on Spirula reticulata OWEN and its phylogeny. In J. M. Hulth (Ed.), Zoologiska Studier. Festskrift W. Lilljeborg tillegnad pah ans attionde fodelsedag af Svenska Zoologer (pp. 99–119). Uppsala.
Lu, C. C. (1998). Order Sepioidea. In P. L. Beesley, G. J. B. Ross, & A. Wells (Eds.), Mollusca: The southern synthesis. Fauna of Australia, part A (Vol. 5, pp. 504–514). Melbourne: CSIRO Publishing.
Lu, C. C., Guerra, A., Palumbo, F., & Summers, W. B. (1992). Order Sepioidea Naef. In: M. J. Sweeney, F. E. R. Roper, K. M. Mangold, M. R. Clarke & S. V. Boletzky (Eds.), “Larval” and juvenile cephalopods: a manual for their identification, Smithsonian Contribution Zoology (vol. 513, pp. 1–282).
Lukeneder, A. (2004). The Olcostephanus level: An upper Valanginian ammonoid mass-occurrence (Lower Cretaceous, Northern Calcareous Alps, Austria). Acta Geolica Polonica, 54, 23–33.
Lukeneder, A., Harzhauser, M., Müllegger, S., & Piller, W. (2008). Stable isotopes (δ18O and δ13C) in Spirula spirula shells from three major oceans indicate developmental changes paralleling depth distributions. Marine Biology, 154, 175–182.
Mehra, A., & Rivin, I. (2010). A real time ocean forecast system for the North Atlantic Ocean. Terrestrial Atmospheric and Oceanic Sciences, 21(1), 211–228.
Mutvei, H. (1964). On the shells of Nautilus and Spirula with notes on the shell secretion in non-cephalopod molluscs. Arkiv för Zoologi, 16, 221–278.
Mutvei, H., & Donovan, D. T. (2006). Siphuncular structure in some fossil coleoids and recent Spirula. Palaeontology, 49, 685–691.
Neige, P., & Warnke, K. (2010). Just how many species of Spirula are there? A morphometric approach. In K. Tanabe, Y. Shigeta, T. Sasaki, & H. Hirano (Eds.), Cephalopods present and past (pp. 77–84). Tokyo: Tokai University Press.
Nesis, K. N. (1987). Cephalopods of the world: Squids, Cuttlefishes, Octopuses and Allies. Neptune City, New Jersey, T.F.H. Publications Inc. Ltd. (translated from Russian).
Nesis, K. N. (1991). Cephalopods of the Benguela upwelling off Namibia. Bulletin of Marine Science, 49(1–2), 199–215.
Nesis, K. N. (1998). Biodiversity and systematics in cephalopods: Unresolved problems require an integrated approach. South African Journal of Marine Science, 20, 165–173.
Nishiguchi, M. K., Lopez, J. E., & Boletzky, S. (2004). Enlightenment of old ideas from new investigations: More regarding the evolution of bacteriogenic light organs in squids. Evolution & Development, 6, 41–49.
Norman, M. (2007). Australian biological resources study. Species Bank. Retrieved January 27, 2015 from http://www.environment.gov.au/cgi-bin/species-bank/sbank-treatment.pl?id=77088.
Norman, M., & Reid, A. (2000). A guide to squid, cuttlefishes and octopuses of Australasia. Victoria: CSIRO Publishing.
Okutani, T. (1995). Cuttlefish and squids of the world in color. Tokyo: National Cooperative Association of Squid Processors.
Owen, R. (1879). Supplementary observations on the anatomy of Spirula australis LAM. Annals and Magazine of Natural History, 13, 1–16.
Perez, J. A. A., Martins, R. S., & Santos, R. A. (2004). Cefalópodes capturados pela pesca comercial de talude no sudeste e sul do Brasil. Notas Técnicas Facimar, 8, 65–74.
Price, G. D., Twitchett, R. J., Smale, C., & Marks, V. (2009). Isotopic analysis of the life history of the enigmatic squid Spirula spirula, with implications for studies of fossil cephalopods. Palaios, 24, 273–279.
Reboulet, S. (2001). Limiting factors on shell growth, mode of life and segregation of Valanginian ammonoid populations: Evidence from adult-size variations. Geobios, 34, 423–435.
Reid, A. (2005). Family Spirulidae Owen, 1936. In P. Jereb & C. F. E. Roper (Eds.), Cephalopods of the World. An Annotated and Illustrated Catalogue of Species known to Date 1 (no. 4, Vol. 1, pp. 211–212). Rome: FAO Species Catalogue for Fishery Purposes.
Reid, A., & Norman, M. D. (1998). Sepiolidae. In K. E. Carpenter & V. H. Niem (Eds.), The living marine resources of the western central Pacific. Cephalopods, crustaceans, holothurians and sharks (Vol. 2, pp. 712–718). Rome: FAO Species Identification Guide for Fishery Purposes.
Santos, R. A., & Haimovici, M. (2002). Cephalopods in the trophic relations off southern Brazil. Bulletin of Marine Science, 71, 753–770.
Schmidt, J. (1922). Live specimens of Spirula. Nature, 110, 788–790.
Warnke, K. (2007). On the species status of Spirula spirula (Linné, 1758) (Cephalopoda): A new approach based on divergence of amino acid sequences between the canaries and New Caledonia. In N. H. Landman, R. A. Davis, & R. H. Mapes (Eds.), Cephalopods present and past: New insights and fresh perspectives (pp. 144–155). Dordrecht: Springer.
Warnke, K., & Keupp, H. (2005). Spirula—A window to the embryonic development of ammonoids? Morphological and molecular indications for a palaeontological hypothesis. Facies, 51, 60–65.
Warnke, K., Oppelt, A., & Hoffmann, R. (2010). Stable isotopes during ontogeny of Spirula and derived hatching temperatures. Ferrantia, 59, 191–201.
Watabe, N. (1988). Shell structure. In E. R. Trueman & M. R. Clarke (Eds.), The mollusca. Form and function (Vol. 11, pp. 69–104). San Diego, CA: Academic Press.
WoRMS Register 2015. World register of marine species. WoRMS taxon details. Retrieved January 16, 2015 from http://www.marinespecies.org/.
Young, R. E., & Sweeney, M. J. (2002). Taxa associated with the family Spirulidae Owen, 1836. Tree of Life, web project. http://tolweb.org/accessory/Spriulidae_Taxa?acc_id=2337.
Acknowledgments
The author is grateful to Deepika Suresh (Centre for Marine Living Resource and Ecology, Kochi, India) for information on fishery data from the Indian Ocean. We thank Elisabeth Haring (Natural History Museum, Vienna, Austria) for fruitful discussions on genetic approaches and for critical comments on the manuscript. Photos were taken by the author and Alice Schumacher (Natural History Museum, Vienna, Austria). We thank the Associate Editor Christian Klug (Palaeontological Institute and Museum, Zurich), René Hoffmann (Institute for Geology, Mineralogy and Geophysics, Bochum) and one anonymous reviewer for their constructive comments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lukeneder, A. New size data on the enigmatic Spirula spirula (Decabrachia, suborder Spirulina), on a global geographic scale. Swiss J Palaeontol 135, 87–99 (2016). https://doi.org/10.1007/s13358-015-0088-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13358-015-0088-8