Abstract
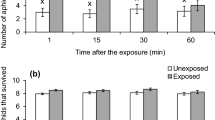
To escape from predators, herbivorous prey could leave their current patch and relocate to an alternative patch. However, when other predators are present on the new patch, prey are again exposed to predation risk. Thus, patch leaving might be affected by the other predators. We studied patch leaving of pea aphids Acyrthosiphon pisum Harris (Hemiptera: Aphididae) in response to ladybird larvae Harmonia axyridis Pallas (Coleoptera: Coccinellidae) on broad bean Vicia faba L. shoots that were offered as patches for aphids. We tested whether shoot leaving was affected by the presence of predators on alternative shoots under laboratory conditions. Odors from alternative shoots were evaluated as possible cues used by aphids to assess predation risk on the shoots. We exposed aphids to odors from alternative shoots with conspecifics plus either adult or larval ladybirds or larval green lacewings Mallada desjardinsi Navas (Neuroptera: Chrysopidae). Shoot leaving was reduced only when adult ladybirds were present on the alternative shoots compared with controls (i.e., no predators on the alternative shoots). Odors of both adult ladybirds and of conspecifics being attacked by ladybird larvae were required for reduced leaving. Hence, predation risks on current and alternative patches might affect the antipredator responses of aphids.





Similar content being viewed by others
References
Agarwala BK, Yasuda H, Kajita Y (2003) Effect of conspecific and heterospecific feces on foraging and oviposition of two predatory ladybirds: role of fecal cues in predator avoidance. J Chem Ecol 29:357–376
Braendle C, Weisser WW (2001) Variation in escape behavior of red and green clones of the pea aphid. J Insect Behav 14:497–509
Canard M (2001) Natural food and feeding habits of lacewings. In: McEwen P, New TR, Whittington AE (eds) Lacewings in the crop environment. Cambridge University Press, Cambridge, pp 116–129
Choh Y, Takabayashi J (2007) Predator avoidance in phytophagous mites: response to present danger depends on alternative host quality. Oecologia 151:262–267
Choh Y, Takabayashi J (2010) Predator avoidance by phytophagous mites is affected by the presence of herbivores in a neighboring patch. J Chem Ecol 36:614–619
Clegg JM, Barlow CA (1982) Escape behaviour of the pea aphid Acyrthosiphon pisum (Harris) in response to alarm pheromone and vibration. Can J Zool 60:2245–2252
Dielenberg RA, McGregor IS (2001) Defensive behavior in rats towards predatory odors: a review. Neurosci Biobehav Rev 25:597–609
Dill LM, Fraser AHG, Roitberg BD (1990) The economics of escape behaviour in the pea aphid, Acyrthosiphon pisum. Oecologia 83:473–478
Du YJ, Poppy GM, Powell W, Pickett JA, Wadhams LJ, Woodcock CM (1998) Identification of semiochemicals released during aphid feeding that attract parasitoid Aphidius ervi. J Chem Ecol 24:1355–1368
Frazer BD (1988) Coccinellidae. In: Minks AK, Harrewijn WH (eds) Aphids: their biology, natural enemies and control, volume B. Elsevier, Amsterdam, pp 231–248
Gish M, Dafni A, Inbar M (2012) Young aphids avoid erroneous dropping when evading mammalian herbivores by combining input from two sensory modalities. PLoS One 7:e32706
Gnanvossou D, Hanna R, Dicke M (2003) Infochemical-mediated intraguild interactions among three predatory mites on cassava plants. Oecologia 135:84–90
Gross P (1993) Insect behavioral and morphological defenses against parasitoids. Annu Rev Entomol 38:251–273
Hatano E, Kunert G, Weisser WW (2010) Aphid wing induction and ecological costs of alarm pheromone emission under field conditions. PLoS One 5:e11188
Hodek I, Evans EW (2012) Food relationships. In: Hodek I, van Emden HF, Honek A (eds) Ecology and behaviour of the ladybird beetles (Coccinellidae). Blackwell, Hoboken, pp 141–168
Ito Y (1989) The evolutionary biology of sterile soldiers in aphids. Trends Ecol Evol 4:69–73
Janssen A, Bruin J, Jacobs G, Schraag R, Sabelis MW (1997) Predators use volatiles to avoid prey patches with conspecifics. J Anim Ecol 66:223–232
Keiser CN, Mondor EB (2015) Cues of predation risk Induce instar- and genotype-specific changes in pea aphid colony spatial structure. Ethology 121:144–151
Kotler BP, Brown JS, Slotow RH, Goodfriend WL, Strauss M (1993) The influence of snakes on the foraging behaviour of gerbils. Oikos 67:309–316
Kunert G, Otto S, Röse USR, Gershenzon J, Weisser WW (2005) Alarm pheromone mediates production of winged dispersal morphs in aphids. Ecol Lett 8:596–603
Kusch RC, Mirza RS, Chivers DP (2004) Making sense of predator scents: investigating the sophistication of predator assessment abilities of fathead minnows. Behav Ecol Sociobiol 55:551–555
Lima SL (1998) Nonlethal effects in the ecology of predator–prey interactions. Bioscience 48:25–34
Lima SL, Dill LM (1990) Behavioral decisions made under the risk of predation: a review and prospectus. Can J Zool 68:619–640
Loose CJ, Dawidowicz P (1994) Trade-offs in diel vertical migration by zooplankton: the costs of predator avoidance. Ecology 75:2255–2263
Losey JE, Denno RF (1998) The escape response of pea aphids to foliar-foraging predators: factors affecting dropping behaviour. Ecol Entomol 23:53–61
Loxdale HD, Lushai G (2007) Population genetic issues: the unfolding story using molecular markers. In: van Emden HF, Harington R (eds) Aphids as crop pests. CAB International, Wallingford, pp 31–68
Luca RM, Gerlai R (2012) In search of optimal fear inducing stimuli: differential behavioral responses to computer animated images in zebrafish. Behav Brain Res 226:66–76
Matsumoto T, Itioka T, Nshida T (2002) Fitness cost of parasitoid avoidance behavior in the arrowhead scale, Unaspis yanonensis Kuwana. Entomol Exp Appl 105:83–88
Miller LR, Gutzke WHN (1999) The role of the vomeronasal organ of crotalines (Reptilia: Serpentes: Viperidae) in predator detection. Anim Behav 58:53–57
Mondor EB, Roitberg BD (2004) Inclusive fitness benefits of scent-marking predators. Proc R Soc B Biol Sci 271:S341–S343
Montgomery ME, Nault LR (1977) Comparative response of aphids to the alarm pheromone, (E)-β-farnesene. Entomol Exp Appl 22:236–242
Muratori FB, Rouyar A, Hance T (2014) Clonal variation in aggregation and defensive behavior in pea aphids. Behav Ecol 25:901–908
Nault LR, Edwards LJ, Styer WE (1973) Aphid alarm pheromones: secretion and reception. Environ Entomol 2:101–105
Nelson EH (2007) Predator avoidance behavior in the pea aphid: costs, frequency, and population consequences. Oecologia 151:22–32
Nelson EH, Matthews SE, Rosenheim JA (2004) Predators reduce prey population growth by inducing changes in prey behavior. Ecology 85:1853–1858
New TR (1988) Neuroptera. In: Minks AK, Harrewijn WH (eds) Aphids: their biology, natural enemies and control, volume B. Elsevier, Amsterdam, pp 249–258
Pestena JLT, Baird DJ, Soares AMVM (2013) Predator threat assessment in Daphnia magna: the role of kairomones versus conspecific alarm cues. Mar Freshw Res 64:679–686
R Development Core Team (2015) R (version 3.3.2), a language and environment for statistical computing. R Foundation, Vienna. http://www.R-project.org
Relyea RA (2003) How prey respond to combined predators: a review and an empirical test. Ecology 84:1827–1839
Roitberg BD, Myers JH (1979) Behavioural and physiological adaptations of pea aphids (Homoptera: Aphididae) to high ground temperatures and predator disturbance. Can Entomol 111:515–519
Schoeppner NM, Relyea RA (2005) Damage, digestion, and defence: the roles of alarm cues and kairomones for inducing prey defences. Ecol Lett 8:505–512
Stamp NE, Bowers MD (1988) Direct and indirect effects of predator wasps (Polistes sp.: Vespidae) on gregarious caterpillars (Hemileuca lucina: Saturniidae). Oecologia 75:619–624
Thaker M, Vanak AT, Owen CR, Ogden MB, Niemann SM, Slotow R (2011) Minimizing predation risk in a landscape of multiple predators: effects on the spatial distribution of African ungulates. Ecology 92:398–407
Tollrian R (1995) Predator-induced morphological defenses: costs, life history shifts, and maternal effects in Daphnia pulex. Ecology 76:1691–1705
Vadas RL, Elner RW (2003) Responses to predation cues and food in two species of sympatric, tropical sea urchins. Mar Ecol (Berl) 24:101–121
Verheggen FJ, Haubruge E, Moraes CMD, Mescher MC (2009) Social enviroment influences aphid production of alarm pheromone. Behav Ecol 20:283–288
Weisser WW, Braendle C, Minoretti N (1999) Predator-induced morphological shift in the pea aphid. Proc R Soc B Biol Sci 266:1175–1181
Witz BW (1989) Antipredator mechanisms in arthropods: a twenty year literature survey. Fla Entomol 73:71–99
Acknowledgements
We thank Masashi Nomura and Kiyoshi Nakamuta for their valuable comments and Masayuki Hayashi and Haruka Kakuta for help with the experiments. This study was supported by the Japan Society for the Promotion of Science (Basic Research C; grant number 23570018 to YC).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tamai, K., Choh, Y. Antipredator response of pea aphids Acyrthosiphon pisum (Hemiptera: Aphididae): effects of predation risks from an alternative patch on a current patch. Appl Entomol Zool 53, 267–274 (2018). https://doi.org/10.1007/s13355-018-0554-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13355-018-0554-z