Abstract
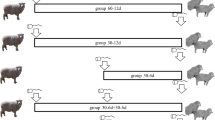
The objectives of this work were to evaluate the in vitro release and in vivo pharmacokinetics and local tolerability of a novel, segmented ethylene-vinyl acetate (EVA) intravaginal ring (IVR) delivering progesterone (P) in drug-naïve ovariectomized female Dorset crossbred sheep. Following preparation and assessment of in vitro release of P, animals were randomized into one of six treatment groups: group 1 Crinone® 8% gel (90 mg); group 2 Prometrium® 200-mg capsules; group 3 placebo IVR; group 4 progesterone (P) IVR 4 mg/day; group 5 P IVR 8 mg/day; or group 6 P IVR 12 mg/day. Crinone 8% gel and Prometrium capsules were administered once daily for 28 days. IVRs were inserted vaginally on day 1 and remained in place through day 14; a new ring was administered on day 15 and was removed at day 28. Animals underwent daily examinations to confirm ring placement, and vaginal irritation was scored from 0 (none) to 4 (severe). Blood samples were taken at scheduled times for pharmacokinetic analysis. Postmortem examinations performed on all IVR groups included vaginal irritation, macroscopic, and microscopic evaluations, including irritation scoring and histopathology. Intravaginal rings were retained over 28 days in all animals. Clinical observations showed no significant abnormal findings in any group. Pharmacokinetic analysis in animals showed sustained release of P over from days 0 through 14 of ring use. Irritation scores and microscopic assessments were consistent with the IVRs being well tolerated. These results will guide future human clinical studies to ultimately develop an IVR for use in women for the prevention of preterm birth.




Similar content being viewed by others
References
Lawn JE, Gravett MG, Nunes TM, Rubens CE, Stanton C, Group tGR. Global report on preterm birth and stillbirth (1 of 7): definitions, description of the burden and opportunities to improve data. BMC Pregnancy Childbirth. 2010;10(1):S1. https://doi.org/10.1186/1471-2393-10-s1-s1.
Preterm birth. Centers for Disease Control and Prevention. 2016. https://www.cdc.gov/reproductivehealth/maternalinfanthealth/pretermbirth.htm. Accessed 13 August 2018 2018.
Infant Mortality. Centers for disease control and prevention. 2016. https://www.cdc.gov/reproductivehealth/maternalinfanthealth/infantmortality.htm. Accessed 13 August 2018 2018.
March of Dimes. Save the children. Born too soon: the global action report on preterm birth. In: Howson C, Kinney M, Lawn J, editors. Geneva: WHO; 2012.
Blanks AM, Brosens JJ. Progesterone action in the myometrium and decidua in preterm birth. Facts Views Vis Obgyn. 2012;4(3):33–43.
Gellersen B, Fernandes MS, Brosens JJ. Non-genomic progesterone actions in female reproduction. Hum Reprod Update. 2009;15(1):119–38. https://doi.org/10.1093/humupd/dmn044.
Caritis SN, Feghali MN, Grobman WA, Rouse DJ. What we have learned about the role of 17-alpha-hydroxyprogesterone caproate in the prevention of preterm birth. Semin Perinatol. 2016;40(5):273–80. https://doi.org/10.1053/j.semperi.2016.03.002.
Fonseca EB, Celik E, Parra M, Singh M, Nicolaides KH. Progesterone and the risk of preterm birth among women with a short cervix. N Engl J Med. 2007;357(5):462–9. https://doi.org/10.1056/NEJMoa067815.
De Franco EA, O'Brien JM, Adair CD, Lewis DF, Hall DR, Fusey S, et al. Vaginal progesterone is associated with a decrease in risk for early preterm birth and improved neonatal outcome in women with a short cervix: a secondary analysis from a randomized, double-blind, placebo-controlled trial. Ultrasound Obstet Gynecol. 2007;30(5):697–705. https://doi.org/10.1002/uog.5159.
Hassan SS, Romero R, Vidyadhari D, et al. Vaginal progesterone reduces the rate of preterm birth with sonographic short cervix: a multicenter, randomized, double-blind,placebo-controlled trial. Ultrasound Obstet Gynecol. 2011;38(1):18–31. https://doi.org/10.1002/uog.9017.
Winer N, Bretelle F, Senat M-V, Bohec C, Deruelle P, Perrotin F, et al. 17 alpha-hydroxyprogesterone caproate does not prolong pregnancy or reduce the rate of preterm birth in women at high risk for preterm delivery and a short cervix: a randomized controlled trial. Am J Obstet Gynecol. 2015;212(4):485.e1-.e10. https://doi.org/10.1016/j.ajog.2014.10.1097.
Grobman WA, Thom EA, Spong CY, Iams JD, Saade GR, Mercer BM, et al. 17 alpha-hydroxyprogesterone caproate to prevent prematurity in nulliparas with cervical length less than 30 mm. Am J Obstet Gynecol. 2012;207(5):390.e1-.e8. https://doi.org/10.1016/j.ajog.2012.09.013.
Kimball AB, Javorsky E, Ron ES, Crowley W, Langer R. A novel approach to administration of peptides in women: systemic absorption of a GnRH agonist via transvaginal ring delivery system. J Control Release. 2016;233:19–28. https://doi.org/10.1016/j.jconrel.2016.04.035.
Draize JH, Woodard G, Calvery HO. Methods for the study of irritation and toxicity of substances applied topically to the skin and mucous membranes. J Pharmacol Exp Ther. 1944;82(3):377–90.
Eckstein P, Jackson MCN, Millman N, Sobrero AJ. Comparison of vaginal tolerance tests of spericidal preparations in rabbits and monkeys. J Reprod Fertil. 1969;20(1):85–93. https://doi.org/10.1530/jrf.0.0200085.
Blencowe H, Cousens S, Chou D, Oestergaard M, Say L, Moller A-B. Born too soon: the global epidemiology of 15 million preterm births. Reprod Health. 2013;10(1):S2. https://doi.org/10.1186/1742-4755-10-s1-s2.
Sipola-Leppänen M, Vääräsmäki M, Tikanmäki M, Matinolli H-M, Miettola S, Hovi P, et al. Cardiometabolic risk factors in young adults who were born preterm. Am J Epidemiol. 2015;181(11):861–73. https://doi.org/10.1093/aje/kwu443.
Tikanmäki M, Tammelin T, Sipola-Leppänen M, Kaseva N, Matinolli H-M, Miettola S, et al. Physical fitness in young adults born preterm. Pediatrics. 2016;137(1):e20151289. https://doi.org/10.1542/peds.2015-1289.
Norman M. Preterm birth and the shape of the heart. Circulation. 2013;127(2):160–1. https://doi.org/10.1161/CIRCULATIONAHA.112.152827.
Romero R, Nicolaides K, Conde-Agudelo A, Tabor A, O'Brien JM, Cetingoz E, et al. Vaginal progesterone in women with an asymptomatic sonographic short cervix in the midtrimester decreases preterm delivery and neonatal morbidity: a systematic review and metaanalysis of individual patient data. Am J Obstet Gynecol. 2012;206(2):124.e1-.e19. https://doi.org/10.1016/j.ajog.2011.12.003.
Conde-Agudelo A, Romero R, Nicolaides K, Chaiworapongsa T, O'Brien JM, Cetingoz E, et al. Vaginal progesterone vs cervical cerclage for the prevention of preterm birth in women with a sonographic short cervix, previous preterm birth, and singleton gestation: a systematic review and indirect comparison metaanalysis. Am J Obstet Gynecol. 2013;208(1):42.e1-.e18. https://doi.org/10.1016/j.ajog.2012.10.877.
Dodd JM, Jones L, Flenady V, Cincotta R, Crowther CA. Prenatal administration of progesterone for preventing preterm birth in women considered to be at risk of preterm birth. Cochrane Database Syst Rev 2013(7). doi:https://doi.org/10.1002/14651858.CD004947.pub3.
Nguyen BT, Jensen JT. Evaluating the efficacy and safety of a progestin- and estrogen-releasing ethylene vinyl acetate copolymer contraceptive vaginal ring. Expert Opin Drug Saf. 2014;13(10):1423–30. https://doi.org/10.1517/14740338.2014.948842.
Roumen FJME, Mishell DR. The contraceptive vaginal ring, NuvaRing®, a decade after its introduction. Eur J Contracept Reprod Health Care. 2012;17(6):415–27. https://doi.org/10.3109/13625187.2012.713535.
Brache V, Faundes A. Contraceptive vaginal rings: a review. Contraception. 2010;82(5):418–27. https://doi.org/10.1016/j.contraception.2010.04.012.
van Laarhoven JAH, Kruft MAB, Vromans H. In vitro release properties of etonogestrel and ethinyl estradiol from a contraceptive vaginal ring. Int J Pharm. 2002;232(1):163–73. https://doi.org/10.1016/S0378-5173(01)00900-0.
Matlin SA, Belenguer A, Hall PE. Progesterone-releasing vaginal rings for use in postpartum contraception. I. In vitro release rates of progesterone from core-loaded rings. Contraception. 1992;45(4):329–41. https://doi.org/10.1016/0010-7824(92)90055-X.
Nath A, Sitruk-Ware R. Progesterone vaginal ring for contraceptive use during lactation. Contraception. 2010;82(5):428–34. https://doi.org/10.1016/j.contraception.2010.05.016.
RamaRao S, Clark H, Merkatz R, Sussman H, Sitruk-Ware R. Progesterone vaginal ring: introducing a contraceptive to meet the needs of breastfeeding women. Contraception. 2013;88(5):591–8. https://doi.org/10.1016/j.contraception.2013.05.004.
Carr SL, Gaffield ME, Dragoman MV, Phillips S. Safety of the progesterone-releasing vaginal ring (PVR) among lactating women: a systematic review. Contraception. 2016;94(3):253–61. https://doi.org/10.1016/j.contraception.2015.04.001.
Dragonis C, Maltaris T, Binder H, Kat M, Mueller A, Cupisti S, et al. Progesterone bioavailability with a progesterone-releasing silicone vaginal ring in IVF candidates. Eur J Med Res. 2007;12:264–7.
Stadtmauer L, Silverberg KM, Ginsburg ES, Weiss H, Howard B. Progesterone vaginal ring versus vaginal gel for luteal support with in vitro fertilization: a randomized comparative study. Fertil Steril. 2013;99(6):1543–9. https://doi.org/10.1016/j.fertnstert.2012.12.052.
Stadtmauer L, Harrison DD, Boyd J, Bocca S, Oehninger S. Pilot study evaluating a progesterone vaginal ring for luteal-phase replacement in donor oocyte recipients. Fertil Steril. 2009;92(5):1600–5. https://doi.org/10.1016/j.fertnstert.2008.08.085.
Stadtmauer L, Waud K. Progesterone vaginal ring for luteal support. J Obstet Gynaecol India. 2015;65(1):5–10. https://doi.org/10.1007/s13224-014-0634-0.
Zegers-Hochschild F, Balmaceda JP, Fabres C, Alam V, Mackenna A, Fernández E, et al. Prospective randomized trial to evaluate the efficacy of a vaginal ring releasing progesterone for IVF and oocyte donation. Hum Reprod. 2000;15(10):2093–7. https://doi.org/10.1093/humrep/15.10.2093.
Romero R, Nicolaides KH, Conde-Agudelo A, O'Brien JM, Cetingoz E, Da Fonseca E, et al. Vaginal progesterone decreases preterm birth ≤ 34 weeks of gestation in women with a singleton pregnancy and a short cervix: an updated meta-analysis including data from the OPPTIMUM study. Ultrasound Obstet Gynecol. 2016;48(3):308–17. https://doi.org/10.1002/uog.15953.
Norman JE, Bennett P. Preterm birth prevention—time to PROGRESS beyond progesterone. PLoS Med. 2017;14(9):e1002391. https://doi.org/10.1371/journal.pmed.1002391.
Acknowledgments
The authors wish to thank QPharma (ring manufacture), MPI Research, a Charles River Company for conducting the animal study, and Pyxant Laboratories for performing the bioanalytical work.
Funding
The financial support for this study was provided by Juniper Pharmaceuticals.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Weiss, H., Martell, B., Constantine, G.D. et al. Pharmacokinetics and tolerability of a novel progesterone intravaginal ring in sheep. Drug Deliv. and Transl. Res. 9, 1008–1016 (2019). https://doi.org/10.1007/s13346-019-00646-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-019-00646-x