Abstract
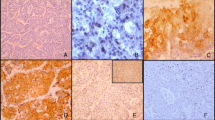
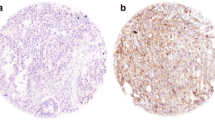
Carcinoma of unknown primary (CUP) is a heterogeneous entity with different clinical and histological features. The aim of this study was to investigate the clinicopathological features and expression of proteins associated with carcinogenesis and tumor environment in different histological subtypes of CUP. Sixty-nine cases of CUP were subjected to immunohistochemistry for EGFR, phospho-EGFR, HER-2, phospho-HER-2, p53, ERCC1, RRM1, REDD1, HIF1α, COX-2, GLUT-1, 14-3-3σ, Phospho-mTOR, Phospho-S6, AMPKα1, Phospho-Akt, PDGF-β receptor, and caveolin-1, and fluorescence in situ hybridization for HER-2 gene amplification. Fourteen (20.3%) cases were poorly differentiated carcinoma, 24 (34.8%) were adenocarcinoma (AD), 17 (24.6%) were squamous cell carcinoma (SC), and 14 (20.3%) were undifferentiated carcinoma (UD). AD were mostly carcinomatosis type, while SC and UD were mostly nodal type (p < 0.001). SC showed more frequent EGFR overexpression (p < 0.001) and Glut-1 (p = 0.001). AD (p = 0.001) and carcinomatosis (p < 0.001) types showed shorter overall survival. SCs expressing Glut-1, HIF1α, and COX2 showed a poor prognosis (p = 0.048, 0.029, and 0.042, respectively). CUP shows various clinicopathological features according to the histological subtypes. SC is mainly associated with nodal metastasis in the head and neck, and frequent EGFR overexpression and Glut-1 expression. Glut-1, HIF1α, and COX2 expression in SC is associated with a poor prognosis.


Similar content being viewed by others
References
Pavlidis N, Fizazi K. Cancer of unknown primary (CUP). Crit Rev Oncol Hematol. 2005;54:243–50.
Haskell CM, Cochran AJ, Barsky SH, Steckel RJ. Metastasis of unknown origin. Curr Probl Cancer. 1988;12:5–58.
Krementz ET, Cerise EJ, Foster DS, Morgan Jr LR. Metastases of undetermined source. Curr Probl Cancer. 1979;4:4–37.
Lembersky BC, Thomas LC. Metastases of unknown primary site. Med Clin North Am. 1996;80:153–71.
Levi F, Te VC, Erler G, Randimbison L, La Vecchia C. Epidemiology of unknown primary tumours. Eur J Cancer. 2002;38:1810–2.
van de Wouw AJ, Janssen-Heijnen ML, Coebergh JW, Hillen HF. Epidemiology of unknown primary tumours; incidence and population-based survival of 1285 patients in southeast netherlands, 1984–1992. Eur J Cancer. 2002;38:409–13.
van de Wouw AJ, Jansen RL, Speel EJ, Hillen HF. The unknown biology of the unknown primary tumour: A literature review. Ann Oncol. 2003;14:191–6.
Pentheroudakis G, Briasoulis E, Karavassilis V, Fountzilas G, Xeros N, Samelis G, et al. Chemotherapy for patients with two favourable subsets of unknown primary carcinoma: Active, but how effective? Acta Oncol. 2005;44:155–60.
Wolff AC, Hammond ME, Schwartz JN, Hagerty KL, Allred DC, Cote RJ, et al. American society of clinical oncology/college of american pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol. 2007;25:118–45.
Pentheroudakis G, Pavlidis N. Perspectives for targeted therapies in cancer of unknown primary site. Cancer Treat Rev. 2006;32:637–44.
Hainsworth JD, Lennington WJ, Greco FA. Overexpression of her-2 in patients with poorly differentiated carcinoma or poorly differentiated adenocarcinoma of unknown primary site. J Clin Oncol. 2000;18:632–5.
Pavlidis N, Briassoulis E, Bai M, Fountzilas G, Agnantis N. Overexpression of c-myc, ras and c-erbb-2 oncoproteins in carcinoma of unknown primary origin. Anticancer Res. 1995;15:2563–7.
Denko NC. Hypoxia, hif1 and glucose metabolism in the solid tumour. Nat Rev Cancer. 2008;8:705–13.
Gatenby RA, Gillies RJ. A microenvironmental model of carcinogenesis. Nat Rev Cancer. 2008;8:56–61.
Moreno-Sanchez R, Rodriguez-Enriquez S, Marin-Hernandez A, Saavedra E. Energy metabolism in tumor cells. FEBS J. 2007;274:1393–418.
Ortega AD, Sanchez-Arago M, Giner-Sanchez D, Sanchez-Cenizo L, Willers I, Cuezva JM. Glucose avidity of carcinomas. Cancer Lett. 2009;276:125–35.
Csiki I, Yanagisawa K, Haruki N, Nadaf S, Morrow JD, Johnson DH, et al. Thioredoxin-1 modulates transcription of cyclooxygenase-2 via hypoxia-inducible factor-1alpha in non-small cell lung cancer. Cancer Res. 2006;66:143–50.
Kaidi A, Qualtrough D, Williams AC, Paraskeva C. Direct transcriptional up-regulation of cyclooxygenase-2 by hypoxia-inducible factor (hif)-1 promotes colorectal tumor cell survival and enhances hif-1 transcriptional activity during hypoxia. Cancer Res. 2006;66:6683–91.
Legan M, Luzar B, Marolt VF. Expression of cyclooxygenase-2, glucose transporter-1 and angiogenesis in gallbladder carcinomas and their impact on prognosis. Scand J Gastroenterol. 2009;44:1101–8.
Eckert AW, Lautner MH, Taubert H, Schubert J, Bilkenroth U. Expression of glut-1 is a prognostic marker for oral squamous cell carcinoma patients. Oncol Rep. 2008;20:1381–5.
Choi YS, Kim SJ, Kim DS, Park SJ, Park Y, Shin HJ, et al. Glucose transporter-1 expression in squamous cell carcinoma of the tongue. Cancer Res Treat. 2007;39:109–15.
Pizzi S, Porzionato A, Pasquali C, Guidolin D, Sperti C, Fogar P, et al. Glucose transporter-1 expression and prognostic significance in pancreatic carcinogenesis. Histol Histopathol. 2009;24:175–85.
Endo M, Tateishi U, Seki K, Yamaguchi U, Nakatani F, Kawai A, et al. Prognostic implications of glucose transporter protein-1 (glut-1) overexpression in bone and soft-tissue sarcomas. Jpn J Clin Oncol. 2007;37:955–60.
Bos R, van der Groep P, Greijer AE, Shvarts A, Meijer S, Pinedo HM, et al. Levels of hypoxia-inducible factor-1alpha independently predict prognosis in patients with lymph node negative breast carcinoma. Cancer. 2003;97:1573–81.
Schindl M, Schoppmann SF, Samonigg H, Hausmaninger H, Kwasny W, Gnant M, et al. Overexpression of hypoxia-inducible factor 1alpha is associated with an unfavorable prognosis in lymph node-positive breast cancer. Clin Cancer Res. 2002;8:1831–7.
Trastour C, Benizri E, Ettore F, Ramaioli A, Chamorey E, Pouyssegur J, et al. Hif-1alpha and ca ix staining in invasive breast carcinomas: Prognosis and treatment outcome. Int J Cancer. 2007;120:1451–8.
Hung JJ, Yang MH, Hsu HS, Hsu WH, Liu JS, Wu KJ. Prognostic significance of hypoxia-inducible factor-1alpha, twist1 and snail expression in resectable non-small cell lung cancer. Thorax. 2009;64:1082–9.
Chan CM, Ma BB, Hui EP, Wong SC, Mo FK, Leung SF, et al. Cyclooxygenase-2 expression in advanced nasopharyngeal carcinoma–a prognostic evaluation and correlation with hypoxia inducible factor 1alpha and vascular endothelial growth factor. Oral Oncol. 2007;43:373–8.
Ogane N, Yasuda M, Shimizu M, Miyazawa M, Kamoshida S, Ueda A, et al. Clinicopathological implications of expressions of hypoxia-related molecules in esophageal superficial squamous cell carcinoma. Ann Diagn Pathol. 2010;14:23–9.
Xu CZ, Dong P, Li XY, Zhang ZJ. Expression and clinical significance of hypoxia-inducible factor-1alpha and cyclooxygenase-2 in laryngeal squamous cell carcinoma. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2009;44:57–62.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Koo, J.S., Kim, H. Hypoxia-related protein expression and its clinicopathologic implication in carcinoma of unknown primary. Tumor Biol. 32, 893–904 (2011). https://doi.org/10.1007/s13277-011-0190-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-011-0190-5