Abstract
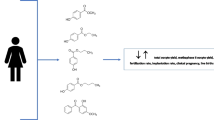
Bisphenol A (BPA) is an endocrine-disrupting compound (EDC) that is used widely in commercial products in the production of polycarbonate plastics for baby and water bottles, epoxy resins for lacquer lining of food and beverage cans and water pipes, dental sealants, dental composites and thermal receipts paper. There is inhibitory effect of BPA on nuclear estrogen (E2) production in granulosa cells of developing follicles that disrupt normal development to the antral follicles via suppression of E2 in granulosa cells of developing follicles during the menstrual cycle followed by reduction in the number of oocytes retrieved in in-vitro fertilization (IVF) patients. Several studies corroborate an inverse association between serum and/or urinary BPA concentration and the IVF outcome: Peak E2 levels and the number of oocytes retrieved. Upon oral ingestion, 99.5% of unconjugated parent BPA (free BPA) is metabolized to either BPA glucuronide (BPA-G) or BPA sulfate (BPA-S). The unconjugated BPA can bind to the estrogen receptors (ER) while conjugated BPA (biologically inactive BPA) do not bind the estrogen receptor (ER). The challenge is to assess the relationship between BPA exposure among infertile patients with respect to follicular response and health during IVF. The establishment of temporal sequence between BPA exposure and infertility would be the research question to answer: Which route is a better biomarker? The advantages of urine BPA collection would provide pragmatic advantages for clinicians in order to practice cost-effective medicine. However, unconjugated BPA measurement (compared to total BPA) introduces challenges in measurement accuracy since unconjugated BPA requires higher magnitude of limit of detection (LOD) with higher risk of contamination from the medical equipment. The difference in route of BPA assessment could introduce bias in the interpretation of results in terms of the association between BPA levels and the number of oocytes. Fujimoto et al. and Bloom et al. analyzed the relationship between serum BPA and IVF outcome in infertile women. It may sound hypothetically justified due to utilizing serum unconjugated BPA, this strategy is not successful in choosing a practical biomarker of BPA exposure due to toxicokinetic properties of BPA metabolism and excretion in humans.
Similar content being viewed by others
References
Vandenberg, L. N. et al. Urinary, circulating, and tissue biomonitoring studies indicate widespread exposure to bisphenol A. Environ Health Perspect 118:1055–1070 (2010).
Brede, C., Fjeldal, P., Skjevrak, I. & Herikstad, H. Increased migration levels of bisphenol A from polycarbonate baby bottles after dishwashing, boiling and brushing. Food Addit Contam 20:684–689 (2003).
Bae, B., Jeong, J. H. & Lee, S. J. The quantification and characterization of endocrine disruptor bisphenol-A leaching from epoxy resin. Water Sci Technol 46: 381–387 (2002).
Sasaki, N. et al. Salivary bisphenol-A levels detected by ELISA after restoration with composite resin. J Mater Sci Mater Med 16:297–300 (2005).
Ehrlich, S. et al. Urinary bisphenol A concentrations and early reproductive health outcomes among women undergoing IVF. Hum Reprod 27:3583–3592 (2012).
Souter, I. et al. The association of bisphenol-A urinary concentrations with antral follicle counts and other measures of ovarian reserve in women undergoing infertility treatments. Reprod Toxicol 42:224–231 (2013).
Crain, D. A. et al. Female reproductive disorders: the roles of endocrine-disrupting compounds and developmental timing. Fertil Steril 90:911–940 (2008).
FAO/WHO. Joint Food and Agriculture Organization of the United Nations and World Health Organization (FAO/WHO) expert meeting to review toxicological and health aspects of bisphenol A: Final report, including report of stakeholder meeting on bisphenol A. Available online at http://apps.who.int/iris/bitstream/10665/ 44624/1/97892141564274_eng.pdf?ua=1 (2011).
National Toxicology Program. US Department of Health and Human Services.NTP-CERHR monograph on the potential human reproductive and developmental effects of bisphenol-A. NTP Brief on Bisphenol-A. Available online at: http://ntp.niehs.nih.gov/ntp/ohat/ bisphenol/bisphenol.pdf (2008).
Hiroi, H. et al. Differential interactions of bisphenol-A and 17 beta-estradiol with estrogen receptor alpha (ER alpha) and ER beta. Endocr J 46:773–778 (1999).
Tarumi, H., Imazato, S., Narimatsu, M., Matsuo, M. & Ebisu, S. Estrogenicity of fissure sealants and adhesive resins determined by reporter gene assay. J Dent Res 79:1838–1843 (2000).
Schafer, T. E. et al. Estrogenicity of bisphenol A and bisphenol A dimethacrylate in vitro. J Biomed Mater Res 45:192–197 (1999).
Hunt, P. A. et al. Bipshenol-A exposure causes meiotic aneuploidy in the female mouse. CurrBiol 13:546–553 (2003).
Can, A., Semiz, O. & Cinar, O. Bipshenol-A induces cell cycle delay and alters centrosome and spindle microtubular organization in oocytes during meiosis. MolHum Reprod 11:389–396 (2005).
Eichenlaub-Ritter, U. et al. Exposure of mouse oocytes to bisphenol-A causes meiotic arrest but not aneuploidy. Mutat Res 651:82–92 (2008).
Ikezuki, Y., Tsutsumi, O., Takai, Y., Kamei, Y. & Taketani, Y. Determination of bisphenol A concentrations in human biological fluids reveals significant early prenatal exposure. Hum Reprod 17:2839–2841 (2002).
Kaddar, N. et al. Development of a radioimmunoassay for the measurement of bisphenol A in biological samples. Anal Chim Acta 645:1–4 (2009).
Wetherill, Y. B. et al. In vitro molecular mechanisms of bisphenol A action. Reprod Toxicol 24:178–198 (2007).
Richter, C. A. et al. In vivo effects of bisphenol A in laboratory rodent studies. Reprod Toxicol 24:199–224 (2007).
Kuiper, G. G. et al. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology 138:863–870 (1997).
Chapin, R. E. et al. NTP-CERHR expert panel report on the reproductive and developmental toxicity of bisphenol A. Birth Defects Res B Dev Reprod Toxicol 83:157–395 (2008).
Lubahn, D. B. et al. Alteration of reproductive function but not prenatal sexual development after insertional disruption of the mouse estrogen receptor gene. Proc Natl Acad Sci USA 90:11162–11166 (1993).
Singh, S. P. et al. Impaired estrogen feedback and infertility in female mice with pituitary-specific deletion of estrogen receptor alpha (ESR1). Biol Reprod 81:488–496 (2009).
Krege, J. H. et al. Generation and reproductive phenotypes of mice lacking estrogen receptor beta. Proc Natl Acad Sci USA 95:15677–15682 (1998).
Reed, B. G. & Carr, B. R. The normal menstrual cycle and the control of ovulation. Endotext [Internet]. Available online at: https://www.ncbi.nlm.nih.gov/books/ NBK279054/ (2015).
Elmlinger, M. W., Kühnel, W. & Ranke, M. B. Reference ranges for serum concentrations of lutropin (LH), follitropin (FSH), estradiol (E2), prolactin, progesterone, sex hormone-binding globulin (SHBG), dehydroepiandrosterone sulfate (DHEAS), cortisol and ferritin in neonates, children and young adults. Clin Chem Lab Med 40:1151–1160 (2002).
Peretz, J., Gupta, R. K., Singh, J., Hernández-Ochoa, I. & Flaws, J. A. Bisphenol A impairs follicle growth, inhibits steroidogenesis, and downregulates rate-limiting enzymes in the estradiol biosynthesis pathway. Toxicol Sc 119:209–217 (2011).
Mlynarcíková, A., Kolena, J., Ficková, M. & Scsuková, S. Alterations in steroid hormone production by porcine ovarian granulosa cells caused by bisphenol A and bisphenol A dimethacrylate. Mol Cell Endocrinol 244:57–62 (2005).
Huang, H. & Leung, L. K. Bisphenol A downregulates CYP19 transcription in JEG-3 cells. Toxicol Lett 189:248–252 (2009).
Kwintkiewicz, J., Nishi, Y., Yanase, T. & Giudice, L. C. Peroxisome proliferator-activated receptor-gamma mediates bisphenol A inhibition of FSH-stimulated IGF-1, aromatase, and estradiol in human granulosa cells. Environ Health Perspect 118:400–406 (2010).
Mok-Lin, E. et al. Urinary bisphenol A concentrations and ovarian response among women undergoing IVF. Int J Androl 33:385–393 (2010).
Bloom, M. S. et al. Bisphenol A exposure reduces the estradiol response to gonadotropin stimulation during in vitro fertilization. Fertil Steril 96:672–677 (2011).
Lee, S. H. et al. Changes in steroid metabolism among girls with precocious puberty may not be associated with urinary levels of bisphenol A. Reprod Toxicol 44: 1–6 (2014).
Fujimoto, V. Y. et al. Serum unconjugated bisphenol A concentrations in women may adversely influence oocyte quality during in vitro fertilization. Fertil Steril 95:1816–1819 (2011).
Caserta, D. et al. The influence of endocrine disruptors in a selected population of infertile women. Gynecol Endocrinol 29:444–447 (2013).
Bloom, M. S. et al. Exploratory assessment of perfluorinated compounds and human thyroid function. Physiol Behav 99:240–245 (2010).
Thayer, K. A. et al. Pharmacokinetics of Bisphenol A in Humans Following a Single Oral Administration. Environ Int 83:107–115 (2015).
Le, H. H., Carlson, E. M., Chua, J. P. & Belcher, S. M. Bisphenol A is released from polycarbonate drinking bottles and mimics the neurotoxic actions of estrogen in developing cerebellar neurons. Toxicol Lett 176:149–156 (2008).
European Food Safety Authority (EFSA). Opinion of the Scientific Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food on a request from the Commission related to 2,2-bis(4-hydroxyphenyl)propane (bisphenol A). EFSA J. 428}:1–75. Available online at: http://www.efsa.europa.eu/en/ efsajournal/pub/428.htm (2006)
Biedermann, S., Tschudin, P., Grob, K. Transfer of bisphenol A from thermal printer paper to the skin. Anal Bioanal Chem 398:571–576 (2010).
Calafat, A. M. et al. Exposure to bisphenol A and other phenols in neonatal intensive care unit premature infants. Environ Health Perspect 117:639–644 (2009).
Lassen, C., Mikkelsen, S. H. & Brandt, U. K. Migration of bisphenol A from cash register receipts and baby dummies. Survey of Chemical Substances in Consumer Products 110 (2011). Available online at: http://www2. mst.dk/udgiv/publications/2011/04/978-87-92708-93-9. pdf
Viñas, R., Goldblum, R. M. & Watson, C. S. Rapid estrogenic signaling activities of the modified (chlorinated, sulfonated, and glucuronidated) endocrine disruptor bisphenol A. Endocr Disruptors 1:e25411 (2013).
Wozniak, A. L., Bulayeva, N. N. & Watson, C. S. Xenoestrogens at picomolar to nanomolar concentrations trigger membrane estrogen receptor-alpha-mediated Ca2+ fluxes and prolactin release in GH3/B6 pituitary tumor cells. Environ Health Perspect 113:431–439 (2005).
Kochukov, M. Y., Jeng, Y. J. & Watson, C. S. Alkylphenol xenoestrogens with varying carbon chain lengths differentially and potently activate signaling and functional responses in GH3/B6/F10 somatomammotropes. Environ Health Perspect 117:723–730 (2009).
Jeng, Y. J. & Watson, C. S. Proliferative and anti-proliferative effects of dietary levels of phytoestrogens in rat pituitary GH3/B6/F10 cells -the involvement of rapidly activated kinases and caspases. BMC Cancer 9:334 (2009).
Jeng, Y. J., Kochukov, M. & Watson, C. S. Combinations of physiologic estrogens with xenoestrogens alter calcium and kinase responses, prolactin release, and membrane estrogen receptor trafficking in rat pituitary cells. Environ Health 9:61 (2010).
Volkel, W., Colnot, T., Csanady, G. A., Filser, J. G. & Dekant, W. Metabolism and kinetics of bisphenol a in humans at low doses following oral administration. Chem Res Toxicol 15:1281–1287 (2002).
Teeguarden, J. G. et al. 24-hour human urine and serum profiles of bisphenol A: Evidence against sublingual absorption following ingestion in soup. Toxicol Appl Pharmacol 228:131–142 (2015).
European Food Safety Authority (EFSA). Public consultation on the draft opinion on bisphenol A (BPA) -exposure assessment. Available online at: http://www. efsa.europa.eu/en/consultations/call/130725.htm (2013).
Mortensen, M. E. et al. Urinary concentrations of environmental phenols in pregnant women in a pilot study of the National Children’s Study. Environ Res 129:32–38 (2014).
Vandenberg, L. N. et al. A round robin approach to the analysis of bisphenol a (BPA) in human blood samples. Environ Health 13:25 (2014).
Vom Saal, F. S. et al. Bisphenol A (BPA) pharmacokinetics with daily oral bolus or continuous exposure via silastic capsules in pregnant rhesus monkeys: Relevance for human exposures. Reprod Toxicol 45:105–116 (2014).
Koch, H. M., Kolossa-Gehring, M., Schroter-Kermani, C., Angerer, J. & Bruning, T. Bisphenol A in 24 h urine and plasma samples of the German Environmental Specimen Bank from 1995 to 2009: a retrospective exposure evaluation. J Expo Sci Environ Epidemiol 22:610–616 (2012).
Calafat, A. M. et al. Misuse of blood serum to assess exposure to bisphenol A and phthalates. Breast Cancer Res 15:403 (2013).
Koch, H. M. & Calafat, A. M. Human body burdens of chemicals used in plastic manufacture. Philos Trans R Soc Lond B Biol Sci 364:2063–2078 (2009).
Vandentorren, S. et al. Bisphenol-A and phthalates contamination of urine samples by catheters in the Elfe pilot study: implications for large-scale biomonitoring studies. Environ Res 111:761–764 (2011).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cho, S.H., Choi, Y., Kim, S.H. et al. Urinary bisphenol A versus serum bisphenol A concentration and ovarian reproductive outcomes among IVF patients: Which is a better biomarker of BPA exposure?. Mol. Cell. Toxicol. 13, 351–359 (2017). https://doi.org/10.1007/s13273-017-0039-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13273-017-0039-0