Abstract
Purpose
The main objectives of this study are to validate a reduced-order model for the estimation of the fractional flow reserve (FFR) index based on blood flow simulations that incorporate clinical imaging and patient-specific characteristics, and to assess the uncertainty of FFR predictions with respect to input data on a per patient basis.
Methods
We consider 13 patients with symptoms of stable coronary artery disease for which 24 invasive FFR measurements are available. We perform an extensive sensitivity analysis on the parameters related to the construction of a reduced-order (hybrid 1D–0D) model for FFR predictions. Next we define an optimal setting by comparing reduced-order model predictions with solutions based on the 3D incompressible Navier–Stokes equations. Finally, we characterize prediction uncertainty with respect to input data and identify the most influential inputs by means of sensitivity analysis.
Results
Agreement between FFR computed by the reduced-order model and by the full 3D model was satisfactory, with a bias (\(\text{FFR} _{{\text {3D}}}- \text{FFR} _{{\text {1D}}{-}{\text {0D}}}\)) of \(-\,0.03\,(\pm\, 0.03)\) at the 24 measured locations. Moreover, the uncertainty related to the factor by which peripheral resistance is reduced from baseline to hyperemic conditions proved to be the most influential parameter for FFR predictions, whereas uncertainty in stenosis geometry had greater effect in cases with low FFR.
Conclusion
Model errors related to solving a simplified reduced-order model rather than a full 3D problem were small compared with uncertainty related to input data. Improved measurement of coronary blood flow has the potential to reduce uncertainty in computational FFR predictions significantly.






Similar content being viewed by others
References
Antiga, L., M. Piccinelli, L. Botti, B. Ene-Iordache, A. Remuzzi, and D. A. Steinman. An image-based modeling framework for patient-specific computational hemodynamics. Med. Biol. Eng. Comput. 46(11):1097–1112, 2008. http://link.springer.com/10.1007/s11517-008-0420-1.
Blanco, P. J., C. A. Bulant, L. O. Müller, G. D. M. Talou, C. G. Bezerra, P. L. Lemos, and R. A. Feijóo. Comparison of 1D and 3D models for the estimation of fractional flow reserve. arXiv:1805.11472 [physics] (2018). ArXiv: 1805.11472.
Boileau, E., S. Pant, C. Roobottom, I. Sazonov, J. Deng, X. Xie, and P. Nithiarasu. Estimating the accuracy of a reduced-order model for the calculation of fractional flow reserve (FFR). Int. J. Numer. Methods Biomed. Eng. 34(1):e2908, 2018. http://doi.wiley.com/10.1002/cnm.2908.
Bråten, A. T., and R. Wiseth. Diagnostic Accuracy of CT-FFR Compared to Invasive Coronary Angiography with Fractional Flow Reserve—Full Text View—ClinicalTrials.gov (2017). https://clinicaltrials.gov/ct2/show/NCT03045601.
Brault, A., L. Dumas, and D. Lucor. Uncertainty quantification of inflow boundary condition and proximal arterial stiffness coupled effect on pulse wave propagation in a vascular network. 2016, arXiv preprint. arXiv:1606.06556.
Cook, C. M., R. Petraco, M. J. Shun-Shin, Y. Ahmad, S. Nijjer, R. Al-Lamee, Y. Kikuta, Y. Shiono, J. Mayet, D. P. Francis, S. Sen, and J. E. Davies. Diagnostic accuracy of computed tomography–derived fractional flow reserve: a systematic review. JAMA Cardiol. 2017. http://cardiology.jamanetwork.com/article.aspx?doi=10.1001/jamacardio.2017.1314.
De Bruyne, B., N. H. Pijls, B. Kalesan, E. Barbato, P. A. Tonino, Z. Piroth, N. Jagic, S. Möbius-Winkler, G. Rioufol, N. Witt, P. Kala, P. MacCarthy, T. Engström, K. G. Oldroyd, K. Mavromatis, G. Manoharan, P. Verlee, O. Frobert, N. Curzen, J. B. Johnson, P. Jüni, and W. F. Fearon. Fractional flow reserve-guided PCI versus medical therapy in stable coronary disease. N. Engl. J. Med. 367(11), 991–1001, 2012. http://www.nejm.org/doi/abs/10.1056/NEJMoa1205361.
Dubin, J., D. C. Wallerson, R. J. Cody, and R. B. Devereux. Comparative accuracy of Doppler echocardiographic methods for clinical stroke volume determination. Am. Heart J. 120(1):116–123, 1990. http://www.sciencedirect.com/science/article/pii/000287039090168W.
Eck, V. G., W. P. Donders, J. Sturdy, J. Feinberg, T. Delhaas, L. R. Hellevik, and W. Huberts. A guide to uncertainty quantification and sensitivity analysis for cardiovascular applications. Int. J. Numer. Methods Biomed. Eng. 2015. http://onlinelibrary.wiley.com/doi/10.1002/cnm.2755/abstract.
Eck, V. G., J. Sturdy, and L. R. Hellevik. Effects of arterial wall models and measurement uncertainties on cardiovascular model predictions. J. Biomech. 2016. http://www.sciencedirect.com/science/article/pii/S0021929016312210.
Evju, Ø., and M. S. Alnæs. CBCFLOW. Bitbucket Repository. 2017.
Feinberg, J., and H. P. Langtangen. Chaospy: an open source tool for designing methods of uncertainty quantification. J. Comput. Sci. 11:46–57, 2015. https://doi.org/10.1016/j.jocs.2015.08.008
Fiorentini, S., L. M. Saxhaug, T. G. Bjastad, and J. Avdal: Maximum velocity estimation in coronary arteries using 3D tracking Doppler. https://doi.org/10.1109/TUFFC.2018.2827241.
Gaur, S., K. A. Øvrehus, D. Dey, J. Leipsic, H. E. Bøtker, J. M. Jensen, J. Narula, A. Ahmadi, S. Achenbach, B. S. Ko, E. H. Christiansen, A. K. Kaltoft, D. S. Berman, H. Bezerra, J. F. Lassen, and B. L. Nørgaard. Coronary plaque quantification and fractional flow reserve by coronary computed tomography angiography identify ischaemia-causing lesions. Eur. Heart J. 37(15), 1220–1227, 2016. https://academic.oup.com/eurheartj/article-lookup/doi/10.1093/eurheartj/ehv690.
Hannawi, B., W. W. Lam, S. Wang, and G. A. Younis. Current use of fractional flow reserve: a nationwide survey. Tex. Heart Inst. J. 41(6):579–584, 2014. https://doi.org/10.14503/THIJ-13-3917.
Holte, E.: Transthoracic Doppler Echocardiography for the Detection of Coronary Artery Stenoses and Microvascular Coronary Dysfunction. NTNU, 2017. https://brage.bibsys.no/xmlui/handle/11250/2486248.
Hunyor, S. N., J. M. Flynn, and C. Cochineas. Comparison of performance of various sphygmomanometers with intra-arterial blood-pressure readings. Br. Med. J. 2(6131):159–162, 1978. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1606220/.
Huo, Y., and G. S. Kassab. Intraspecific scaling laws of vascular trees. J. R. Soc. Interface 9(66), 190–200, 2012. https://doi.org/10.1098/rsif.2011.0270.
Itu, L., P. Sharma, V. Mihalef, A. Kamen, C. Suciu, and D. Lomaniciu. A patient-specific reduced-order model for coronary circulation. In: 2012 9th IEEE International Symposium on Biomedical Imaging (ISBI). IEEE, 2012, pp. 832–835. http://ieeexplore.ieee.org/xpls/abs_all.jsp?arnumber=6235677.
Johnson, N. P., D. T. Johnson, R. L. Kirkeeide, C. Berry, B. De Bruyne, W. F. Fearon, K. G. Oldroyd, N. H. J. Pijls, and K. L. Gould. Repeatability of fractional flow reserve despite variations in systemic and coronary hemodynamics. JACC Cardiovasc. Interv. 8(8):1018–1027, 2015. http://www.sciencedirect.com/science/article/pii/S1936879815006998.
Johnson, N. P., R. L. Kirkeeide, and K. L. Gould. Is discordance of coronary flow reserve and fractional flow reserve due to methodology or clinically relevant coronary pathophysiology? JACC Cardiovasc. Imaging 5(2):193–202, 2012. https://doi.org/10.1016/j.jcmg.2011.09.020
Jones, E., T. Oliphant, P. Peterson, et al. SciPy: open source scientific tools for Python (2001–). http://www.scipy.org/.
Kenner, T.: The measurement of blood density and its meaning. Basic Res. Cardiol. 84(2):111–124, 1989. http://link.springer.com/article/10.1007/BF01907921.
Kim, H. J., I. E. Vignon-Clementel, J. S. Coogan, C. A. Figueroa, K. E. Jansen, and C. A. Taylor. Patient-specific modeling of blood flow and pressure in human coronary arteries. Ann. Biomed. Eng. 38(10):3195–3209, 2010. https://doi.org/10.1007/s10439-010-0083-6
Liang, F., K. Fukasaku, H. Liu, and S. Takagi. A computational model study of the influence of the anatomy of the circle of Willis on cerebral hyperperfusion following carotid artery surgery. Biomed. Eng. Online 10:84, 2011. https://doi.org/10.1186/1475-925X-10-84
Logg, A., K. A. Mardal, and G. Wells, eds. Automated Solution of Differential Equations by the Finite Element Method. Lecture Notes in Computational Science and Engineering, vol. 84. Berlin: Springer, 2012. https://doi.org/10.1007/978-3-642-23099-8.
Mantero, S., R. Pietrabissa, and R. Fumero. The coronary bed and its role in the cardiovascular system: a review and an introductory single-branch model. J. Biomed. Eng. 14(2):109–116, 1992. http://linkinghub.elsevier.com/retrieve/pii/014154259290015D.
Matsuda, J., T. Murai, Y. Kanaji, E. Usui, M. Araki, T. Niida, S. Ichijyo, R. Hamaya, T. Lee, T. Yonetsu, M. Isobe, and T. Kakuta. Prevalence and clinical significance of discordant changes in fractional and coronary flow reserve after elective percutaneous coronary intervention. J. Am. Heart Assoc. 2016. https://doi.org/10.1161/JAHA.116.004400.
Morris, P. D., D. A. Silva Soto, J. F. Feher, D. Rafiroiu, A. Lungu, S. Varma, P. V. Lawford, D. R. Hose, and J. P. Gunn. Fast virtual fractional flow reserve based upon steady-state computational fluid dynamics analysis. JACC Basic Transl. Sci. 2(4):434–446, 2017. http://linkinghub.elsevier.com/retrieve/pii/S2452302X17301353.
Mortensen, M., and K. Valen-Sendstad. Oasis: a high-level/high-performance open source Navier–Stokes solver. Comput. Phys. Commun. 188:177–188, 2015. http://linkinghub.elsevier.com/retrieve/pii/0010465514003786.
Murray, C. D. The physiological principle of minimum work. Proc. Natl Acad. Sci. USA 12(3):207–214, 1926. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1084489/.
Otsuki, T., S. Maeda, M. Iemitsu, Y. Saito, Y. Tanimura, R. Ajisaka, and T. Miyauchi. Systemic arterial compliance, systemic vascular resistance, and effective arterial elastance during exercise in endurance-trained men. Am. J. Physiol. Regul. Integr. Comp. Physiol. 295(1):R228–R235, 2008. http://www.physiology.org/doi/10.1152/ajpregu.00009.2008.
Pijls, N. H., W. F. Fearon, P. A. Tonino, U. Siebert, F. Ikeno, B. Bornschein, M. van’t Veer, V. Klauss, G. Manoharan, T. Engstrøm, K. G. Oldroyd, P. N. Ver Lee, P. A. MacCarthy, and B. De Bruyne. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention in patients with multivessel coronary artery disease. J. Am. Coll. Cardiol. 56(3):177–184, 2010. http://linkinghub.elsevier.com/retrieve/pii/S0735109710016025.
Ri, K., K. K. Kumamaru, S. Fujimoto, Y. Kawaguchi, T. Dohi, S. Yamada, K. Takamura, Y. Kogure, N. Yamada, E. Kato, R. Irie, T. Takamura, M. Suzuki, M. Hori, S. Aoki, and H. Daida. Noninvasive computed tomography-derived fractional flow reserve based on structural and fluid analysis: reproducibility of on-site determination by unexperienced observers. J. Comput. Assist. Tomogr. 1, 2017. http://Insights.ovid.com/crossref?an=00004728-900000000-99330.
Robert, C. P., and G. Casella. Monte Carlo Statistical methods, 2nd edn., Softcover Reprint of the Hardcover 2, 2004 edn. Springer Texts in Statistics. New York: Springer, 2010.
Rogers, G., and T. Oosthuyse. A comparison of the indirect estimate of mean arterial pressure calculated by the conventional equation and calculated to compensate for a change in heart rate. Int. J. Sports Med. 21(02):90–95, 2000. https://www.thieme-connect.com/products/ejournals/html/10.1055/s-2000-8865?update=true#R616-17.
Sakamoto, S., S. Takahashi, A. U. Coskun, M. I. Papafaklis, A. Takahashi, S. Saito, P. H. Stone, and C. L. Feldman. Relation of distribution of coronary blood flow volume to coronary artery dominance. Am. J. Cardiol. 111(10):1420–1424, 2013. http://inkinghub.elsevier.com/retrieve/pii/S000291491300386X.
Saltelli, A.: Making best use of model evaluations to compute sensitivity indices. Comput. Phys. Commun. 145(2):280–297, 2002. https://doi.org/10.1016/S0010-4655(02)00280-1
Saltelli, A.: Global Sensitivity Analysis: The Primer. Chichester Wiley, 2008. http://catalog.lib.ncsu.edu/record/NCSU2123570.
Sankaran, S., H. J. Kim, G. Choi, and C. A. Taylor. Uncertainty quantification in coronary blood flow simulations: impact of geometry, boundary conditions and blood viscosity. J. Biomech. 49(12):2540–2547, 2016. http://www.sciencedirect.com/science/article/pii/S0021929016000117.
Schroeder, W. J., and K. M. Martin. The visualization toolkit. In: Visualization Handbook. Elsevier, 2005, , pp. 593–614. http://linkinghub.elsevier.com/retrieve/pii/B9780123875822500320.
Shahzad, R., H. Kirişli, C. Metz, H. Tang, M. Schaap, L. van Vliet, W. Niessen, and T. van Walsum. Automatic segmentation, detection and quantification of coronary artery stenoses on CTA. Int. J. Cardiovasc. Imaging 29(8):1847–1859, 2013. https://doi.org/10.1007/s10554-013-0271-1
Simo, J., and F. Armero. Unconditional stability and long-term behavior of transient algorithms for the incompressible Navier–Stokes and Euler equations. Comput. Methods Appl. Mech. Eng. 111(1–2):111–154, 1994. http://linkinghub.elsevier.com/retrieve/pii/0045782594900426.
Smith, N., A. Pullan, and P. Hunter. An anatomically based model of transient coronary blood flow in the heart. SIAM J. Appl. Math. 62(3):990–1018, 2002. https://epubs.siam.org/doi/abs/10.1137/S0036139999355199.
Spaan, J. A. E.: Coronary blood flow. In: Developments in Cardiovascular Medicine, vol. 124. Dordrecht: Springer, 1991. http://link.springer.com/10.1007/978-94-011-3148-3.
Antiga, L., and S. Manini. The vascular modeling toolkit website. https://www.vmtk.org. Accessed 27 Oct 2017.
Sturdy, J., J. K. Kjernlie, H. M. Nydal, V. G. Eck, and L. R. Hellevik. Uncertainty of computational coronary stenosis assessment and model based mitigation of image resolution limitations (Forthcoming).
Tonino, P. A., B. De Bruyne, N. H. Pijls, U. Siebert, F. Ikeno, M. vant Veer, V. Klauss, G. Manoharan, T. Engstrøm, K. G. Oldroyd, et al. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N. Engl. J. Med. 360(3):213–224, 2009. http://www.nejm.org/doi/full/10.1056/NEJMoa0807611.
Uren, N. G., J. A. Melin, B. De Bruyne, W. Wijns, T. Baudhuin, and P. G. Camici. Relation between myocardial blood flow and the severity of coronary-artery stenosis. N. Engl. J. Med. 330(25):1782–1788, 1994. https://doi.org/10.1056/NEJM199406233302503
Wongkrajang, P., W. Chinswangwatanakul, C. Mokkhamakkun, N. Chuangsuwanich, B. Wesarachkitti, B. Thaowto, S. Laiwejpithaya, and O. Komkhum. Establishment of new complete blood count reference values for healthy Thai adults. Int. J. Lab. Hematol. https://onlinelibrary.wiley.com/doi/abs/10.1111/ijlh.12843.
World Health Organization. Top 10 Causes of Death, 2018. http://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death.
Xiao, N., J. Alastruey, and C. Alberto Figueroa. A systematic comparison between 1-D and 3-D hemodynamics in compliant arterial models. Int. J. Numer. Methods Biomed. Eng. 30(2):204–231, 2014. https://doi.org/10.1002/cnm.2598
Young, D. F., and F. Y. Tsai. Flow characteristics in models of arterial stenoses—I. Steady flow. J. Biomech. 6(4):395–410, 1973. http://linkinghub.elsevier.com/retrieve/pii/0021929073900997.
Yushkevich, P. A., J. Piven, H. C. Hazlett, R. G. Smith, S. Ho, J. C. Gee, and G. Gerig. User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. NeuroImage 31(3):1116–1128, 2006. http://linkinghub.elsevier.com/retrieve/pii/S1053811906000632.
Zienkiewicz, O. C., R. L. Taylor, and P. Nithiarasu. The Finite Element Method for Fluid Dynamics, 7th edn. Oxford: Butterworth-Heinemann, 2014. OCLC: ocn869413341.
Acknowledgments
This work was partially supported by NTNU Health (Strategic Research Area at the Norwegian University of Science and Technology) and by The Liaison Committee for Education, Research and Innovation in Central Norway. Computational resources in Norwegian HPC Infrastructure were granted by the Norwegian Research Council by Project Nr. NN9545K. LRH was partly funded by a Peder Sather Grant: Mainstreaming Sensitivity Analysis And Uncertainty Auditing.
Conflict of interest
There are no conflicts of interest.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Institutional and/or National Research Committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editors Dr. David A. Steinman, Dr. Francesco Migliavacca, and Dr. Ajit P. Yoganathan oversaw the review of this article.
Appendices
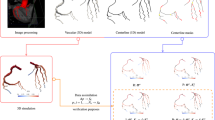
Appendix 1: Reduced-Order Model: from a 3D Domain to a 1D Network
After centerline generation, the portions of the centerlines which coincide with arterial junctions were masked in order to exclude them from the 1D domain definition, since no reasonable 1D description of such portions of the domain can be formulated. We used the VMTK scripts ‘vmtkbranchextractor’ and ‘vmtkcenterlinemerge’ to obtain the topology of the 1D network. The first script uses definitions on what is treated as bifurcations in order to split the centerline into segments. The second script connects centerlines at a single point at bifurcations.1 However, we did not find that these methods sufficiently split the domain into 1D segments, i.e. normally too little is considered as junction, see left part of Fig. 8. And thus additional processing was performed as described below. Points in mother (m) and daughter vessels (d) were masked based on different criteria. Points \(p_{\text {d},1}\) and \(p_{\text {d},2}\) in daughter vessel 1 and daughter vessel 2 were considered as part of the junction if
where \(S_{\text {d}_{1}-\text {d}_{2}}\) is the distance between points \(p_{\text {d},1}\) and \(p_{\text {d},2}\) and \(r_{{\text {max-sphere}},\text {d}_{1}}\) and \(r_{{\text {max-sphere}},\text {d}_{2}}\) are the maximum inscribed sphere radius at points \(p_{\text {d},1}\) and \(p_{\text {d},2}\) respectively. A point \(p_{\text {m}}\) of the mother vessel was considered as part of the junction if
where \(S_{\text {m}-\text {d}}\) is the distance between point \(p_{\text {m}}\) and a point, \(p_{\text {d}}\) in a daughter vessel. The value \(r_{\text {max-sphere},\text {d}_{\text {m}}}\) for a point \(p_{\text {d}}\) situated n points downstream of the center of the junction is found by evaluating \(r_{\text {max-sphere}}\) for all daughter vessels at the same number of points downstream the center of the junction, and taking the minimum observed value, see Fig. 7. The criteria in Eqs. (21) and (22) were designed to keep as much of the 1D–0D domain intact; however, this caused incomplete masking in some cases (particularly of centerline points in daughter vessels) as visualized in Fig. 8. In order to check for the smoothness of transition from junctions to 1D segments we calculated the ratio of the maximum inscribed sphere and radius of the cross-sectional area, \(r_{i}=\sqrt{\frac{A_{i}}{\pi }}\) for successive points, i.e.
After the initial junction mask in step 1, \(\gamma\) was calculated for the next 10 downstream centerline points in daughter vessels. If \(\gamma\) exceeded a value of 1.3 for a centerline point, \(i=n,\) the centerline points \(1,\ldots ,n,\, n<10\) were also marked as part of the junction. \(\gamma\) represents the ratio of the maximum inscribed sphere and the radius of the cross-sectional area for successive points. As noted earlier, this value is normally high in the transition from a bifurcation to an arterial segment. However this value may also be relatively high at the start/end of non cylindrical segments. Having observed values of up to approximately 1.3 in such regions, we used this value as a threshold. If we had used a lower value, non cylindrical segments could be misinterpreted as part of junctions. Figure 8 show the result before (left) and after (right) the correction.
Appendix 2: 3D Modeling Framework: Model Setting and Results
Mathematical Model
We consider the domain defined by the coronary tree vessels as \(\Upomega _{\text {f}} \in {\mathbb {R}}^{3}.\) Moreover, its boundary is partitioned as \(\partial \Upomega _{\text {f}} :=\Upgamma _{\text {in}} \cup \Upsigma \cup \Upgamma _{\text {out}},\) where \(\Upsigma\) represents the wall boundary, \(\Upgamma _{\text {in}}\) is the inlet cross-section and \(\Upgamma _{\text {out}} = \bigcup \nolimits _{j=1}^{N_{\text {out}}} \Upgamma _{{{out}},j}\) is the union of the \(N_{\text {out}}\) outlets of the tree. Furthermore, blood flow in coronary arteries is modeled assuming that blood is an incompressible Newtonian fluid, for which the incompressible Navier–Stokes equations hold. These equations, along with boundary conditions are given by
where \(\rho =1.05\,{\text {g}}/{\text {cm}}^{3}\) is the blood density and \(\nu\) is the kinematic viscosity, given by \(\nu =\mu /\rho ,\) with blood viscosity \(\mu =0.035 P.\) Furthermore, \(P_{\text {in}}(t)\) is a prescribed pressure function and \(P_{{out},j}(t)\) is provided by the lumped-parameter model that is coupled to the jth outlet. Coronary arteries experience increased impedance during systole due to the contraction of the myocardium and increased pressure in the left ventricle. To account for this effect each outlet is coupled to a lumped-parameter model,24 which in turn derives from the original work by Mantero et al.27 This lumped-parameter model setup is depicted in Fig. 9 and the variation of volume V of each compartment is governed by the ordinary differential equations (ODEs)
Here, P and R represent pressure and resistance of different elements of the lumped model. Moreover, subscripts p, m and d stand for proximal, medial and distal, respectively. Volumes relate to pressure via compliance C by the following relation
where \(P_{\text {LV}}\) is the left ventricular pressure.
Schematic representation of a lumped-parameter model coupled to the jth 3D domain outlet \(\Upgamma ^{\text {out}}_{j},\) shown in grey, and related to Eqs. (25) and (26). Portion of the 3D domain is also shown. C is compliance, R is resistance and P is pressure. Moreover, subscripts p, m and d stand for proximal, medial and distal, respectively. \(P_{\text {LV}}\) represents the time-varying left ventricular pressure.
Flow \(Q_{{out},j},\) for the jth outlet, is computed as
where \({\mathbf {n}}_{{out},j}\) is the exterior unit-vector normal for \(\Upgamma _{{out},j}.\) On the other hand, \(P_{{out},j}\) is provided by the lumped-parameter model as
Numerical Methods
The mathematical models presented in the “Mathematical Model” section are solved using the open-source library CBCFLOW,11 based on FEniCS.26 In particular, CBCFLOW provides a flexible problem setup, allowing to combine its highly efficient incompressible Navier–Stokes solver with typical boundary conditions and simple models used in computational hemodynamics. Here, a Python script allowed CBCFLOW to interact with lumped-parameter models and to prescribe the needed boundary conditions.
The problem defined by Eq. (24) is solved by CBCFLOW using the Incremental Pressure Correction Scheme, described in Ref. 43. The computational mesh is composed of tetrahedral elements. The velocity field is approximated using piecewise-quadratic polynomials, while linear polynomials are used for pressure. The solver implementation follows very closely the one reported in Ref. 30. Apart from spatial and temporal discretization, the only numerical parameter to be set for this scheme is a multiplicative factor for the streamline diffusion stabilization term, referred to as s in this work, see Ref. 55 for details about this term. Moreover, Eq. (25) are solved using an explicit Euler discretization. Numerical parameters are set to \(\Updelta t= 1\,{\text {ms}}\) and \(s=1.\) A parameter independence study has shown that such choices provide parameter independent FFR predictions for a set of patient-specific geometries.
Definition of Main Parameters from Patient-Specific Simulations
The flow to the coronary branch is based on the work of Sakamoto et al.,37 who studied the dependence of flow on coronary branch dominance. From this we calculate the relative distribution of total coronary flow, \(\gamma ^{j}_{k},\) to each coronary branch j for k dominant vasculature (\(j=\{{\text {right branch}},{\text {left branch}}\}\) and \(k=\{{\text {left dominance}},{\text {right dominance}}\}\)). Furthermore, total coronary flow was assumed to be \(4.5\%\) of CO. Thus, the baseline coronary flow to a specific branch is
The two flow fractions may be combined to get the fraction of CO to a branch, \(\lambda _{\text {cor}}=0.045\cdot \gamma ^{j}_{k}.\)
Total peripheral compliance is computed as a percentage of total arterial compliance of 1.7 mL mmHg.32 The percentage of the total arterial compliance assigned to the left/right branch is equal to the relation between flow in branch of interest over total CO, that is
MAP, pulse pressure (PP) and cardiac cycle duration (T) are extracted from pressure tracings for aortic pressure acquired during invasive FFR measurement. MAP is computed by time-averaging the pressure signal over a cardiac cycle. MAP and PP are used to prescribe a scaled characteristic aortic pressure waveform at the network’s inlet and a scaled characteristic left ventricle pressure waveform (peak left ventricle pressure is 1.05 times the peak inlet pressure) for all lumped-parameter models. We chose to use a characteristic waveform as opposed to the measured waveform since the latter is not perfectly periodic and a corresponding left ventricle pressure waveform would then have to be estimated/fitted. The characteristic waveforms, taken from Ref. 24, are shown in Fig. 10. Total peripheral resistance for a given branch is estimated from MAP and the target branch flow in baseline conditions \(q^{j}_{\text {cor}}\) as
where \(P_{\text {d}} = 5\,{\text {mmHg}}\) is the outflow/venous pressure.
Aortic and left ventricle characteristic waveforms used for patient-specific simulations. \(\tau\) and \({\tilde{p}}\) are normalized time and pressure. The waveform shapes were taken from Ref. 24.
The total peripheral resistance \(R_{\text {tot}}\) and total peripheral compliance \(C_{\text {tot}}\) are distributed among outlets using Murray’s law,31 that is
and
where j stands for the jth outlet of the network. \(R_{j}\) and \(C_{j}\) have to be subsequently distributed among the different compartments (see Fig. 9) of the lumped-parameter model attached to the jth outlet. The fractions for distributing \(R_{j}\) among \(R_{p,j},\)\(R_{m,j}\) and \(R_{ d,j}\) are set to 0.01, 0.84 and 0.15, respectively. Similarly, fractions used to distribute \(C_{j}\) among \(C_{a,j}\) and \(C_{m,j}\) are 0.025 and 0.975, respectively. Parameter distribution among components of lumped-parameter models were empirically determined to obtain diastolic coronary flow rate waveforms with appropriate pulsatility.
Modeling Pipeline
The modeling pipeline is as follows
-
(1)
Using parameters defined in the previous section as initial guess, total peripheral resistance \(R_{\text {tot}}\) for a given branch is modified in order to match target branch flow \(q_{\text {cor}}\) defined by Eq. (29). The iterative procedure is described later in this section.
-
(2)
We determine heart rate, MAP and PP from pressure tracings taken under hyperemic conditions. Moreover, we use \(R_{\text {tot}}\) from previous step to estimate a new total peripheral resistance, now in hyperemic conditions: \(R_{{\text {tot}},{\text {hyp}}} = R_{\text {tot}}/\alpha ,\) which is subsequently distributed among outlets with criteria specified in Eq. (32). The hyperemic factor, \(\alpha ,\) was set to 3.
-
(3)
Steady state simulations are performed prescribing \(\text{MAP}\) at the inlet and replacing the lumped-parameter model by a single resistance at each outlet, where such resistance is \(R_{j}=R_{{p},j}+R_{{m},j}+R_{{d},j},\) as for the transient case.
Solution Monitoring and Total Resistance Estimation
As noted previously, total peripheral resistance \(R_{\text {tot}}\) is modified in order to match average branch flow \(q_{\text {cor}}\) defined in Eq. (29). Starting with the initial guess provided by Eq. (31), \(R_{\text {tot}}\) is updated after each cardiac cycle using
where m is the iteration index (which corresponds to the cardiac cycle index), \(q_{\text {cor}}\) is the target coronary flow in a branch, provided by Eq. (29) and \(q_{\text {obs}}\) is the observed flow at the branch inlet. \(\omega\) is a relaxation parameter and was set to \(\omega = 0.9.\) Once a new value for \(R_{\text {tot}}\) is available, the resistance is distributed among outlets using Eq. (32).
In order to computed predicted FFR, we have to extract average pressure values form 3D solutions. To do so we first define the subdomain
where \({\mathbf {x}}_{k}\) is the k-th node of a vessel’s centerline that corresponds to the point where the invasive FFR measurement was taken, and \(r_{k}\) is the radius of the vessel at node k. Locations \({\mathbf {x}}_{k}\) were identified by inspection of angiograms and segmentation results by modelers and cardiologists. Next, average pressure (over space and time) is computed as
3D Simulation Results
While the primary objective of this work is to present and analyze a 1D–0D framework for model-based FFR prediction, we report here the comparison of predicted FFR values obtained using the 3D modeling framework described in B vs. invasively measured FFR values. Such values are provided in order to show that the FFR prediction modeling framework, while still under development, provides results that are aligned with many publications on model-based FFR prediction. The average error of FFR predictions was \(-\,0.033\) and the standard deviation of the error was 0.119. Moreover, the correlation coefficient of predicted FFR vs. invasive FFR was 0.84. In terms of diagnostic accuracy, prediction sensitivity, specificity, positive predicted value and negative predicted value were 60, 93, 86 and \(76\%,\) respectively. Figure 11 shows a scatter plot and a Bland–Altman plot for predicted FFR vs. measured FFR. More relevant for the current study are results reported in Fig. 12, that shows predicted FFR based on steady state simulations vs. predicted FFR based on transient simulations. In this case, mean error was \(-\,0.004\) and standard deviation of the error was 0.003, with a correlation coefficient of 1.00.
Predicted FFR based on steady state simulations, \({{ {\text{FFR}}}} _{{\text {3D}},{\text {SS}}},\) vs. predicted FFR based on transient simulations, \({{ {\text{FFR}}}} _{{\text {3D}},{\text {US}}}.\) Scatter plot with grey line showing the FFR cutoff value of 0.8 (left) and Bland–Altman plot with dashed lines showing ± 2 standard deviations (right).
Appendix 3: UQ&SA Framework
We summarize the framework used by treating the patient specific model as a function \(M\) that predicts \(y =M (\mathbf {z})\) based on input data \(\mathbf {z}.\) As the input data is uncertain it is represented as a random variable \(\mathbf {Z}\) which implies that prediction \(Y\) is also an uncertain random variable. We employ the nonintrusive UQ&SA methods of Monte Carlo and polynomial chaos to characterize the distribution of Y given the distribution of \(\mathbf {Z}.\) This is achieved by evaluating \(M\) at many samples drawn from the distribution of \(\mathbf {Z},\) i.e. \(y =M (\mathbf {z})\) at each sample point in \(\left\{ \mathbf {z} ^{(s)}\right\} _{s=1}^{N_{\text {s}}}\). Eck et al.9 present several methods and concepts of UQ&SA within the context of cardiovascular modeling, and we refer the reader to this work for more details regarding the methods of UQ&SA used here.
The uncertainty of \(Y\) is fundamentally due to the uncertainty of \(\mathbf {Z}\) propagated through the model \(M.\) Thus it is critical to employ a distribution of \(\mathbf {Z}\) that reflects the conditions the UQ&SA is intended to analyze. To assess performance of a patient specific model the input distribution must reflect the actual uncertainties present in clinical procedures and population variation. However, UQ&SA may also be employed to analyze a model’s range of behavior and to identify parameters relevant for estimation from measured values of \(y.\) In this case the distribution of \(\mathbf {Z}\) should reflect the range of plausible values for the inputs. Typically, only a range of values is considered and no prior knowledge is available to prioritize certain regions, thus a uniform distribution is appropriate to investigate the model’s dependence on the parameters.
Once the approximate distribution of \(Y\) is available from the UQ&SA procedure various measures of uncertainty of \(Y\) are available such as statistical moments or quantiles of \(Y.\). These quantities are of primary interest when assessing model performance, however, SA augments this by assessing the portion of uncertainty due to particular inputs, allowing prioritization of efforts to reduce uncertainty. In this context, Sobol sensitivity indices, first-order (\(S_{i}\)) and total (\(S_{{T},i}\)), are widely employed,39 and defined as
where the vector, \({\mathbf {Z}} _{\lnot {{i}}},\) contains all elements of \(\mathbf {Z}\) except \(Z_{i}.\) These indices partition the total \({{\mathbb {V}}\left[ Y \right] }\) into portions attributable to specific combinations of inputs. The first order indices \(S_{i}\) quantify the variance due to \(Z_{i}\) alone, i.e. independent of the values of the other inputs. The total sensitivity index, \(S_{{T},i}\), includes effect due to interaction with other parameters and represents the reduction in variance expected to be achieved by fixing \(Z_{i}\) at a particular value.
Larger values of \(S_{i}\) suggest that \(Z_{i}\) strongly affects \(Y\) and thus may be a prime target for improved measurement or optimization in the context of parameter estimation. In the case where \(S_{{T},i}\) and thus also \(S_{i}\) are small, \(Z_{i}\) has little influence on \(Y\) and should not be prioritized for improved measurement and may not be estimated accurately in an inverse problem context. When \(S_{i}\) is small but \(S_{{T},i}\) is large, the effect of \(Z_{i}\) depends greatly on the values of other parameters thus it may still be valuable to improve its measurement, and it may be estimated in an inverse problem though its identifiability may depend on the values of other parameters.
Appendix 4: Patient-Specific Data and Invasive Measurements
Table 11 shows patient characteristics and non-invasive measurements for all patients considered in this work. Furthermore, Table 12 shows data obtained during invasive angiography, and include stenosis location, quantitative coronary angiography (QCA), cardiac cycle averaged pressure proximal and distal to the stenosis, and heart rate. QCA is a measure of the percent diameter stenosis degree obtained by inspection of angiography images. Figures 13, 14 and 15 shows visualizations of all 3D domains for the patients considered in this work, where we have highlighted the locations of distal measurements with (magenta) spheres, and the branch inlets (orange) where proximal measurements were obtained.
Visualization of 3D domain for some of the patients included in the study (see figure sub-captions for details). Magenta sphere is where distal pressure \(\bar{P}_{\text {distal}}\) was evaluated in 3D domains using Eq. (36). Branch inlet, where proximal pressure \(\bar{P}_{\text {proximal}}\) is prescribed is highlighted in orange. The centerline used as reduced-order model domain is evidenced in blue. (a) Patient 1, left branch (b) Patient 2, left branch (c) Patient 3, left branch (d) Patient 4, right branch (e) Patient 5, left branch (f) Patient 5, right branch (g) Patient 7, left branch (h) Patient 7, right branch.
Visualization of 3D domain for some of the patients included in the study (see figure sub-captions for details). Magenta sphere is where distal pressure \(\bar{P}_{\text {distal}}\) was evaluated in 3D domains using Eq. (36). Branch inlet, where proximal pressure \(\bar{P}_{\text {proximal}}\) is prescribed is highlighted in orange. The centerline used as reduced-order model domain is evidenced in blue. (a) Patient 6, right branch (b) Patient 9, left branch (c) Patient 8, left branch (d) Patient 8, right branch (e) Patient 10, left branch (f) Patient 10, right branch (g) Patient 11, left branch (h) Patient 13, left branch.
Visualization of 3D domain for some of the patients included in the study (see figure sub-captions for details). Magenta sphere is where distal pressure \(\bar{P}_{\text {distal}}\) was evaluated in 3D domains using Eq. (36). Branch inlet, where proximal pressure \(\bar{P}_{\text {proximal}}\) is prescribed is highlighted in orange. The centerline used as reduced-order model domain is evidenced in blue. (a) Patient 12, left branch (b) Patient 12, right branch.
Rights and permissions
About this article
Cite this article
Fossan, F.E., Sturdy, J., Müller, L.O. et al. Uncertainty Quantification and Sensitivity Analysis for Computational FFR Estimation in Stable Coronary Artery Disease. Cardiovasc Eng Tech 9, 597–622 (2018). https://doi.org/10.1007/s13239-018-00388-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13239-018-00388-w