Abstract
In the present paper, a green method was applied for the synthesis of SrAl2O4 nanostructures with the aid of microwave irradiation and pomegranate juice. SrAl2O4 nanocrystals were obtained when the raw materials were irradiated with 720–900 W for 6–10 min and then calcinated at 550 °C for 5 h. Using pomegranate juice as a dispersion and stabilizing agent, SrAl2O4 nanoparticles have been made with better properties in view of morphology and particle size. Also, the effect of some parameters affecting synthesis process such as microwave power and reaction time on the morphology and particle size of product was studied and optimized. X-ray diffraction and field emission-scanning electron microscopy were used to study and characterize the manufactured SrAl2O4 nanoparticles.
Similar content being viewed by others
Introduction
Within the energy-saving materials, there is a class called “long lasting phosphors”. They have the ability to adsorb sunshine and store its energy, and afterwards release the saved solar energy as observable light. So, they can have afterglow for a long time in the dark environment (Wang et al. 2015; Gutiérrez-González et al. 2017). These unique features have attracted extensive attentions because of their wide utilizations in various fields such as warning marks, textile industry, dial plates of glowing watch, automobile components, ship and other apparatus (Chen et al. 2012).
In recent years, Sr-based aluminate materials (Sr n Al n O n ), as suitable host agents, have been attracted much interest due to their unique properties including high chemical stability, excellent quantum efficiency, long lasting afterglow, supreme luminescence characteristic, etc. (Yu et al. 2014; Farzaneh et al. 2017). SrAl2O4 is one of the most usable Sr-based aluminate materials that has numerous desired properties such as good mechanical and thermal persistence, low surface acidity and superior ductility and diffusion nature. Because of these unique features, SrAl2O4 was used as high temperature materials (Tzing and Tuan 1996), diodes (Chen et al. 2013) and AC-LEDs (Li et al. 2015), but its most important application is as catalyst or catalyst support.
The main and conventional synthesis method of strontium aluminate phosphors is the solid-state reaction which has some disadvantages such as long time of sintering and high temperature. Furthermore, the produced particles have relatively large size and crushing the hard phosphor blocks to small particles is very difficult. These properties decrease the luminescence intensity (Zhu et al. 2009). For this reason and at the same time with the improvement of technology, different kinds of chemical synthesis methods have been used for the preparation of strontium aluminate phosphors, such as chemical precipitation(Chen et al. 2008), solvothermal co-precipitation (Xue et al. 2013), response surface (Wang et al. 2016), and top-down (Zhang et al. 2014). In comparison with other methods, microwave synthesis technique is an attractive and beneficial method to prepare SrAl2O4 nanoparticles to produce pure and ultrafine powders at low temperature. Therefore, in the present study, we try to build SrAl2O4 nanostructures by microwave method. Also, SrAl2O4 can dope with rare earth elements (e.g., Eu, Yb, Cr and Dy) as the activator and co-activator and employ as the matrix material (Timilsina et al. 2013; Teng et al. 2014).
In this study, we focus our attention on the reduction of temperature on the synthesis of single-phase nanosized SrAl2O4 with high specific surface area by application of microwave technique. We use pomegranate juice as a well disperser and stabilizer for the green synthesis of SrAl2O4 nanoparticles. Also, the characterization of the produced nanostructures powder and the effect of irradiation time and power on the morphology and size of SrAl2O4 nanoparticles were carried out using X-ray diffraction (XRD) and field emission-scanning electron microscopy (FE-SEM).
Experimental
Reagents and standards
All chemicals had the highest purity and there was no need to purify methods. Strontium (II) nitrate (Sr(NO3)2), aluminium nitrate nanohydrate (Al(NO3)3·9H2O), sodium borohydride (NaBH4) and sodium hydroxide (NaOH) were purchased from Merck (http://www.merckmillipore.com). Also, ethanol (C2H5OH) was prepared from Sigma-Aldrich (http://www.sigmaaldrich.com). Throughout all experiments, deionized water was applied.
Instrumentation
The nanoparticles synthesis was performed by a Feller MWF 2580 FW microwave-oven (http://www.fellergermany.de). Weighing processes were done with an Ohaus AV 246 C balance (http://www.ohaus.com). Memmert oven (http://www.memmert.com) was used for drying the synthesized nanoparticles. An Alfa heater-stirrer (http://www.alfaelectric.com) was applied for the temperature adjustment and stirring the solutions. A Sahand Teb Aria 88-2750 centrifuge was used for faster separation of precipitates from the aqueous phase. Field emission-scanning electron microscopy (FE-SEM) images were obtained with a Hitachi S-4160 instrument (http://www.hitachi-hightech.com). X-ray diffraction (XRD) patterns were recorded by a Philips-X’pertpro apparatus (http://www.panalytical.com) using Ni-filtered Cu Kα (λ = 1.5406 Å) radiation.
Synthesis of SrAl2O4 nanoparticles
1.00 g Sr(NO3)2 and 3.55 g Al(NO3)3·9H2O with a mixture containing 25 ml ethanol and 50 ml deionized water were transferred into a 250 mL beaker and stirred. At the same time, 0.01 g sodium borohydride was added to this solution. Then, sodium hydroxide (2.0 mol L−1) was added dropwise to form precipitates. This mixture was stirred for 15 min, transferred to microwave digestion system with 100% power (900 W) and intermittent program (30 s off and 1 min on) for 6 min and then was allowed to cool to room temperature naturally. After complete sedimentation, the supernatant phase was removed; the precipitates were washed several times using ethanol and deionized water in sequence to eliminate the impurities and centrifuged. This product was dried in an oven (T = 60 °C) for 4 h.
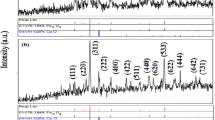
For calcination process, after drying step, the precipitates were transferred to a furnace with 550 °C for 5 h. The morphological studies on final produced SrAl2O4 nanoparticles were performed by application of XRD (Fig. 1) and FE-SEM (Fig. 2a–f). Accordance between XRD pattern and the library of X-ray diffractometer confirmed that the obtained pattern belongs to SrAl2O4 nanoparticles.
Results and discussion
XRD patterns of the produced nanoparticles after further heating at 550 °C are shown in Fig. 1. The crystallinity of prepared SrAl2O4 nanostructures via the mentioned synthesis method can be approved from this XRD pattern. The calcined products at 550 °C showed orthorhombic SrAl2O4 that is only crystalline phase. By application of XRD data, the crystallite diameter (Dc) of the prepared SrAl2O4 nanoparticles those calcined at 550 °C for 5 h, was calculated as 65 nm using the Scherer equation (Jenkins and Snyder 1996):
where K is a constant and equal to 0.9; λ is the wavelength of X-ray source that is used in XRD and equal to 1.54 Å, \(\theta\) is the Bragg angle and β is the pure diffraction broadening of a peak at half-height.
Also, the effect of some parameters affecting on the synthesis process such as irradiation time and power and application of pomegranate juice was investigated and optimized.
Effect of irradiation time and power
The effect of irradiation time and power has been investigated by SEM on the morphology of synthesized SrAl2O4 nanostructures using microwave method. The raw materials were irradiated as intermittent program: 30 s off and 1 min on. Other experiment steps and circumstances are the same as the mentioned synthesis method. The irradiation time and power have been varied between 6, 8 and 10 min at 900 W and 6 and 8 min at 720 W (Table 1). The obtained SEM images of samples are presented in Fig. 2a (900 W and 6 min), b (900 W and 8 min), c (900 W and 10 min), d (720 W and 6 min) and e (720 W and 8 min). As can be seen from the SEM images of Fig. 2a–c, with increasing the irradiation time from 6 to 10 min, the size of the synthesized nanoparticles was decreased. This tendency is also true for Fig. 2d, 2e. In Fig. 2a, the synthesized nanoparticles have a better morphological shape, but their sizes were increased. Also, Fig. 2e, 2d shows that despite the size decreasing with power diminution, the morphological shape and uniformity were improved.
Effect of pomegranate juice on SrAl2O4 morphology
In the recent decade, using green methods for nanoparticles synthesis has attracted more interest, because they are fast, low cost and environmentally friendly techniques. Some of the herbal and fruit essences were applied as stabilizer for control of crystal growth. For this reason, we have used pomegranate juice to investigate the stabilizing and dispersing effect on the morphology of SrAl2O4 nanoparticles. So, the synthesis method was performed as previously presented, except that instead of sodium borohydride addition, 4 droplets of pomegranate juice were added into stirring solution. Other steps were done exactly as presented before and, finally, irradiation was applied with 900 W and 8 min. As can be seen from Fig. 2f, the SEM image of the synthesized SrAl2O4 nanoparticles shows that they have better shape and more uniform distribution. Also, the size of the manufactured nanostructures was decreased in comparison with previous products without pomegranate juice. So, pomegranate juice can apply as a stabilizer and disperser in green synthesis method of SrAl2O4 nanoparticles.
Conclusion
In the present work, SrAl2O4 nanoparticles have been successfully synthesized via a green microwave method. By application of pomegranate juice both as stabilizing and dispersion agent, SrAl2O4 nanoparticles were produced with smaller size, more uniform distribution and better shape. Also, the effect of time and power of microwave irradiation were investigated on the morphological shape and particle size of SrAl2O4 nanostructures. X-ray diffraction (XRD) and field emission-scanning electron microscopy (FE-SEM) were used to complete morphological studies and the obtained data confirmed the orthorhombic structure of synthesized SrAl2O4 nanoparticles without any sign of impurities.
References
Chen J, Gu F, Li C (2008) Influence of precalcination and boron-doping on the initial photoluminescent properties of SrAl2O4:Eu,Dy phosphors. Cryst Growth Des 8:3175–3179
Chen L, Zhang Y, Liu F, Luo A, Chen Z, Jiang Y, Chen S, Liu R-S (2012) A new green phosphor of SrAl2O4:Eu2+, Ce3+, Li+ for alternating current driven light-emitting diodes. Mater Res Bull 47:4071–4075
Chen L, Zhang Y, Xue S, Deng X, Anqi, Luo, Liu F, Jiang Y, Chen S, Bahader A (2013) The green phosphor SrAl2O4:Eu2+, R3+ (R=Y, Dy) and its application in alternating current light-emitting diodes. Funct Mater Lett 06:1350047
Farzaneh F, Kashani Maleki M, Rashtizadeh E (2017) Expedient catalytic access to Knöevenagel condensation using Sr3Al2O6 nanocomposite in room temperature. J Cluster Sci 28:3253–3263
Gutiérrez-González CF, Pozhidaev S, Rivera S, Peretyagin P, Solís W, Díaz LA, Fernandez A, Torrecillas R (2017) Longer-lasting Al2O3-SiCw-TiC cutting tools obtained by spark plasma sintering. Int J Appl Ceram Technol 14:367–373
Jenkins R, Snyder RL (1996) Chemical analysis: introduction to X-ray powder diffractometry. Wiley, New York, p 90
Li B, Zhang J, Zhang M, Long Y, He X (2015) Effects of SrCl2 as a flux on the structural and luminescent properties of SrAl2O4:Eu2+, Dy3+ phosphors for AC-LEDs. J Alloys Compd 651:497–502
Teng Y, Zhou J, Nasir Khisro S, Zhou S, Qiu J (2014) Persistent luminescence of SrAl2O4:Eu2+,Dy3+,Cr3+ phosphors in the tissue transparency window. Mater Chem Phys 147:772–776
Timilsina S, Lee KH, Jang IY, Kim JS (2013) Mechanoluminescent determination of the mode I stress intensity factor in SrAl2O4: Eu2+, Dy3+. Acta Mater 61:7197–7206
Tzing W, Tuan W (1996) The strength of duplex Al2O3–ZnAl2O4 composite. J Mater Sci Lett 15:1395–1396
Wang C, Xuan T, Liu J, Li H, Sun Z (2015) Long Afterglow SrAl2O4:Eu2+,Dy3+ phosphors as luminescent down-shifting layer for crystalline silicon solar cells. Int J Appl Ceram Technol 12:722–727
Wang H, Liang X, Liu K, Zhou Q, Chen P, Wang J, Li J (2016) Synthesis of SrAl2O4:Eu2+ phosphors co-doped with Dy3+, Tb3+, Si4+ and optimization of co-doping amount by response surface method. Opt Mater 53:94–100
Xue Z, Deng S, Liu Y, Lei B, Xiao Y, Zheng M (2013) Synthesis and luminescence properties of SrAl2O4:Eu2+,Dy3+ hollow microspheres via a solvothermal co-precipitation method. J Rare Earths 31:241–246
Yu A, Zhang D, Hu Y, Yang R (2014) Synthesis and characterizations of Dy3+ doped Sr3Al2O6:Eu2+ powder phosphors through citric acid precursor. J Mater Sci 25:4434–4438
Zhang H, Xue Z, Lei B, Dong H, Zhang H, Deng S, Zheng M, Liu Y, Xiao Y (2014) A top-down method to fabricate SrAl2O4:Eu2+,Dy3+ nanosheets from commercial blocky phosphors. Opt Mater 36:1802–1807
Zhu Y, Zheng M, Zeng J, Xiao Y, Liu Y (2009) Luminescence enhancing encapsulation for strontium aluminate phosphors with phosphate. Mater Chem Phys 113:721–726
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Riahi-Madvaar, R., Taher, M.A. & Fazelirad, H. Green and microwave synthesis of SrAl2O4 nanoparticles by application of pomegranate juice: study and characterization. Appl Nanosci 7, 913–917 (2017). https://doi.org/10.1007/s13204-017-0628-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13204-017-0628-1