Abstract
Background
Microbiome modulation is a pillar intervention to treat metabolic syndrome, prestages, and cascade of related pathologies such as atherosclerosis, among others. Lactobacillus and Bifidobacterium probiotic strains demonstrate efficacy to reduce obesity, dyslipidemia, and improve metabolic health. Novel prebiotic substances composed with known probiotics may strongly synergize health benefits to the host. The aim of this study was to evaluate beneficial effects of Lactobacillus and Bifidobacterium strains (probiotics) if composed with nanoceria (potential prebiotic) to reduce cholesterol levels and restore gut microbiota in obese mice.
Materials and Methods
Two lines of mice were used in the study: BALB/c mice (6–8 weeks, 18–24 g) and CBA mice (11–12 months, 20–26 g); experimental animals were fed by fat-enriched diet 3 weeks before the evaluation. Animals were divided into groups to test probiotic strains and nanoceria. All groups received probiotic strains orally and cerium dioxide orally or intravenously in various composition. A group of untreated animals was used as a control. Cholesterol level and gut microbiota of mice were studied.
Results
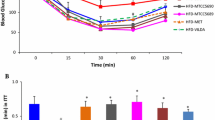
Cerium dioxide nanoparticles, probiotic strain L. casei ІМV В-7280, and composition B. animalis VKB/B. animalis VKL applied separately and in different combinations all reduced at different levels free and bound cholesterol in blood serum of mice fed by fat-enriched diet. The combination of 0.01 M nanoceria and probiotic strain L. casei ІМV В-7280 resulted in the fastest cholesterol level decrease in both young and mature animals. Oral administration of CeO2 applied alone reduced the number of microscopic fungi in the gut of mice and Gram-positive cocci (staphylococci and/or streptococci). Application of L. casei IMV B-7280 as a probiotic strain increased most significantly the number of lactobacilli and bifidobacteria in the gut of mice. The most significant normalization of gut microbiota was observed after oral administration of alternatively either L. casei IMV B-7280 + 0.1 M CeO2 or L. casei IMV B-7280 + 0.01 M CeO2.
Conclusion
Dietary application of nanoceria combined with probiotic strains L. casei IMV B-7280, B. animalis VKB, and B. animals VKL has significantly reduced both free and bound cholesterol levels in serum. Simultaneous administration of probiotics and cerium nanoparticles as a prebiotic, in various combinations, significantly enhanced positive individual effects of them on the gut microbiota spectrum. The presented results provide novel insights into mechanisms behind nutritional supplements and open new perspectives for application of probiotics combined with substances demonstrating prebiotic qualities benefiting, therefore, the host health. Follow-up translational measures are discussed to bring new knowledge from lab to the patient. If validated in a large-scale clinical study, this approach might be instrumental for primary and secondary prevention in obese individual and patients diagnosed with diabetes. To this end, individualized prediction and treatments tailored to the person are strongly recommended to benefit the health condition of affected individuals.
Similar content being viewed by others
References
Duarte AA, Mohsin S, Golubnitschaja O. Diabetes care in figures: current pitfalls and future scenario. EPMA J. 2018;9(2):125–31. https://doi.org/10.1007/s13167-018-0133-y.
World Health Organization (2018). Obesity and overweight. Fact sheet. https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight. (Accessed 25.10.2019).
World Health Organization (2017). Cardiovascular diseases (CVDs). Fact sheet. https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds). (Accessed 25.10.2019).
Golubnitschaja O, Costigliola V, EPMA. General report & recommendations in predictive, preventive and personalised medicine 2012: white paper of the European Association for Predictive, Preventive and Personalised Medicine. EPMA J. 2012;3(1):14. https://doi.org/10.1186/1878-5085-3-14.
Golubnitschaja O, Baban B, Boniolo G, Wang W, Bubnov R, Kapalla M, et al. Costigliola V. Medicine in the early twenty-first century: paradigm and anticipation - EPMA position paper 2016. EPMA J. 2016;7:23. https://doi.org/10.1186/s13167-016-0072-4.
Golubnitschaja O, Costigliola V, EPMA. EPMA summit 2014 under the auspices of the presidency of Italy in the EU: professional statements. EPMA J. 2015;6(1):4. doi: 10.1186/s13167-015-0026-2.
Bubnov RV, Spivak MY, Lazarenko LM, Bomba A, Boyko NV. Probiotics and immunity: provisional role for personalized diets and disease prevention. EPMA J. 2015;6:14.
Ndumele CE, Nasir K, Conceiçao RD, Carvalho JAM, Blumenthal RS, Santos RD. Hepatic steatosis, obesity, and the metabolic syndrome are independently and additively associated with increased systemic inflammation. Arterioscler Thromb Vasc Biol. 2011;31(8):1927–32. https://doi.org/10.1161/ATVBAHA.111.228262.
Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute scientific statement. Crit Pathw Cardiol. 2005;4(4):198–203.
Srikanthan K, Feyh A, Visweshwar H, Shapiro JI, Sodhi K. Systematic review of metabolic syndrome biomarkers: a panel for early detection, management, and risk stratification in the West Virginian Population. Int J Med Sci. 2016;13(1):25–38. https://doi.org/10.7150/ijms.13800.
Reid G, Abrahamsson T, Bailey M, Bindels LB, Bubnov R, Ganguli K, et al. How do probiotics and prebiotics function at distant sites? Benefic Microbes. 2017;20:1–14. https://doi.org/10.3920/BM2016.0222.
Bogiatzi C, Gloor G, Allen-Vercoe E, Reid G, Wong RG, Urquhart BL, et al. Metabolic products of the intestinal microbiome and extremes of atherosclerosis. Atherosclerosis. 2018;273:91–7. https://doi.org/10.1016/j.atherosclerosis.2018.04.015.
Reijnders D, Goossens GH, Hermes GD, Neis EP, van der Beek CM, Most J, et al. Effects of gut microbiota manipulation by antibiotics on host metabolism in obese humans: a randomized double-blind placebo-controlled trial. Cell Metab. 2016;24(1):63–74. https://doi.org/10.1016/j.cmet.2016.06.016.
Park S, Bae JH. Probiotics for weight loss: a systematic review and meta-analysis. Nutr Res. 2015;35:566–75.
Jobin C. Precision medicine using microbiota. Science. 2018;359(6371):32–4. https://doi.org/10.1126/science.aar2946.
Bubnov RV, Babenko LP, Lazarenko LM, Mokrozub VV, Demchenko OA, Nechypurenko OV, et al. Comparative study of probiotic effects of Lactobacillus and Bifidobacteria strains on holesterol levels, liver morphology and the gut microbiota ino bese mice. EPMA J. 2017;8(4):357–76. https://doi.org/10.1007/s13167-017-0117-3.
Babenko LP, Sokolviak OY, Мokrozub VV, Nechypurenko OO, Demchenko OM, Bubnov RV, et al. Lactobacillus and Bifidobacterium probiotic strains reduce cholesterol levels and affect the gut microbiota in obese mice. United European Gastroenterol J. 2016;4(5S):A374–5. https://doi.org/10.1177/2050640616663689.
Bubnov RV, Babenko LP, Lazarenko LM, Mokrozub VV, Spivak MY. Specific properties of probiotic strains: relevance and benefits for the host. EPMA J. 2018. https://doi.org/10.1007/s13167-018-0132-z.
Wu Y, Zhang Q, Ren Y, Ruan Z. Effect of probiotic Lactobacillus on lipid profile: a systematic review and meta-analysis of randomized, controlled trials. PLoS One. 2017;12(6):e0178868. https://doi.org/10.1371/journal.pone.0178868.
Bendali F, Kerdouche K, Hamma-Faradji S, Drider D. In vitro and in vivo cholesterol lowering ability of Lactobacillus pentosus KF923750. Benefic Microbes. 2017;8(2):271–80. https://doi.org/10.3920/BM2016.0121.
Song M, Park S, Lee H, Min B, Jung S, Park S, et al. Effect of Lactobacillus acidophilus NS1 on plasma cholesterol levels in diet-induced obese mice. J Dairy Sci. 2015;98(3):1492–501. https://doi.org/10.3168/jds.2014-8586.
Kumar M, Nagpal R, Kumar R, Hemalatha R, Verma V, Kumar A, et al. Cholesterol-lowering probiotics as potential biotherapeutics for metabolic diseases. Exp Diabetes Res. 2012;2012:902917. https://doi.org/10.1155/2012/902917.
Michael DR, Davies TS, Moss JWE, Calvente DL, Ramji DP, Marchesi JR, et al. The anti-cholesterolaemic effect of a consortium of probiotics: an acute study in C57BL/6J mice. Sci Rep. 2017;7(1):2883. https://doi.org/10.1038/s41598-017-02889-5.
Greany KA, Bonorden MJ, Hamilton-Reeves JM, McMullen MH, Wangen KE, Phipps WR, et al. Probiotic capsules do not lower plasma lipids in young women and men. Eur J Clin Nutr. 2008;62:232–7.
Shimizu M, Hashiguchi M, Shiga T, Tamura HO, Mochizuki M. Meta-analysis: effects of probiotic supplementation on lipid profiles in normal to mildly hypercholesterolemic individuals. PLoS One. 2015;10(10):e0139795.
Kristensen NB, Bryrup T, Allin KH, Nielsen T, Hansen TH, Pedersen O. Alterations in fecal microbiota composition by probiotic supplementation in healthy adults: a systematic review of randomized controlled trials. Genome Med. 2016;8(1):52. https://doi.org/10.1186/s13073-016-0300-5.
Garcia SL, Buck M, McMahon KD, Grossart HP, Eiler A, Warnecke F. Auxotrophy and intrapopulation complementary in the ‘interactome’ of a cultivated freshwater model community. Mol Ecol. 2015;24(17):4449–59. https://doi.org/10.1111/mec.13319.
Garcia SL, Stevens SLR, Crary B, Martinez-Garcia M, Stepanauskas R, Woyke T, et al. Contrasting patterns of genome-level diversity across distinct co-occurring bacterial populations. ISME J. 2017;12:742–55. https://doi.org/10.1038/s41396-017-0001-0.
Papadimitriou K, Zoumpopoulou G, Foligné B, Alexandraki V, Kazou M, Pot B, et al. Discovering probiotic microorganisms: in vitro, in vivo, genetic and omics approaches. Front Microbiol. 2015;6:58. https://doi.org/10.3389/fmicb.2015.00058.
Fijan S. Microorganisms with claimed probiotic properties: an overview of recent literature. Int J Environ Res Public Health. 2014;11(5):4745–67.
van den Nieuwboer M, Browne PD, Claassen E. Patient needs and research priorities in probiotics: a quantitative KOL prioritization analysis with emphasis on infants and children. Pharma Nutri. 2016;4(1):19–28.
Lyu Q, Hsu C-C. Can diet influence our health by altering intestinal microbiota-derived fecal metabolites? mSystems. 2018;3(2):e00187–17. https://doi.org/10.1128/mSystems.00187-17.
Malhotra A, Redberg RF, Meier P. Saturated fat does not clog the arteries: coronary heart disease is a chronic inflammatory condition, the risk of which can be effectively reduced from healthy lifestyle interventions. Br J Sports Med. 2017;51:1111–2.
Malhotra A, Noakes T, Phinney S. It is time to bust the myth of physical inactivity and obesity: you cannot outrun a bad diet. Br J Sports Med. 2015;49:967–8.
Bhaskaran K, Dos-Santos-Silva I, Leon DA, Douglas IJ, Smeeth L. Association of BMI with overall and cause-specific mortality: a population-based cohort study of 3·6 million adults in the UK. Lancet Diabetes Endocrinol. 2018;6(12):944–53. https://doi.org/10.1016/S2213-8587(18)30288-2.
Golubnitschaja O. Flammer syndrome in the global context – the “U-Shape” of health risks. Flammer syndrome – from phenotype to associated pathologies, prediction, prevention and personalisation. Springer 2019, ISBN 978-3-030-13549-2 ISBN 978-3-030-13550-8 (eBook), https://doi.org/10.1007/978-3-030-13550-8.
Bubnov R and Golubnitschaja O. Flammer syndrome, disordered eating and microbiome: interrlations, complexity of risks and individual outcomes. In book. Flammer syndrome – from phenotype to associated pathologies, prediction, prevention and personalisation. Springer 2019, ISBN 978-3-030-13549-2 ISBN 978-3-030-13550-8 (eBook), https://doi.org/10.1007/978-3-030-13550-8
Gibson GR, Roberfroid MB. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J Nutr. 1995;125:1401–12.
Gibson GR, Hutkins R, Sanders ME, Prescott SL, Reimer RA, Salminen SJ, et al. Expert consensus document: the International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat Rev Gastroenterol Hepatol. 2017 Aug;14(8):491–502. https://doi.org/10.1038/nrgastro.2017.75.
Marco ML, Heeney D, Binda S, Cifelli CJ, Cotter PD, Foligné B, et al. Health benefits of fermented foods: microbiota and beyond. Curr Opin Biotechnol. 2017 Apr;44:94–102. https://doi.org/10.1016/j.copbio.2016.11.010].
Hunter PM, Hegele RA. Functional foods and dietary supplements for the management of dyslipidaemia. Nat Rev Endocrinol. 2017;13(5):278–88. https://doi.org/10.1038/nrendo.2016.210.
Konopelniuk VV, Goloborodko II, Ishchuk TV, Synelnyk TB, Ostapchenko LI, Spivak MY, et al. Efficacy of Fenugreek-based bionanocomposite on renal dysfunction and endogenous intoxication in high-calorie diet-induced obesity rat model—comparative study. EPMA J. 2017;8:377–90. https://doi.org/10.1007/s13167-017-0098-2.
Spivak MY, Bubnov RV, Yemets IM, Lazarenko LM, Tymoshok NO, Ulberg ZR. Development and testing of gold nanoparticles for drug delivery and treatment of heart failure: a theranostic potential for PPP cardiology. EPMA J. 2013;4(1):20 -10.1186/1878-5085-4-20.
Nido SA, Shituleni SA, Mengistu BM, Liu Y, Khan AZ, Gan F, et al. Effects of selenium-enriched probiotics on lipid metabolism, antioxidative status, histopathological lesions, and related gene expression in mice fed a high-fat diet. Biol Trace Elem Res. 2016;171(2):399–409. https://doi.org/10.1007/s12011-015-0552-8.
Yu I-T, Ju C-C, Lin J, Wu H-L, Yen H-T. Effects of probiotics and selenium combination on the immune and blood cholesterol concentration of pigs. J Anim Feed Sci. 2004;13(4):625–34. https://doi.org/10.22358/jafs/67630/2004.
Zholobak NM, Sherbakov AB, Babenko LS, Bogorad-Kobelska OS, Bubnov RV, Ivanov VK, et al. The perspectives of biomedical application of the nanoceria. EPMA J. 2014;5(Suppl 1):A136–2. https://doi.org/10.1186/1878-5085-5-S1-A136.
Beregova TV, Neporada KS, Skrypnyk M, Falalyeyeva TM, Zholobak NM, Shcherbakov OB, et al. Efficacy of nanoceria for periodontal tissues alteration in glutamate-induced obese rats-multidisciplinary considerations for personalized dentistry and prevention. EPMA J. 2017;8(1):43–9. https://doi.org/10.1007/s13167-017-0085-7.
Zholobak NM, Ivanov VK, Shcherbakov AB, Shaporev AS, Polezhaeva OS, Baranchikov AY, et al. UV-shielding property, photocatalytic activity and photocytotoxicity of ceria colloid solutions. J Photochem Photobiol B. 2011;102(1):32–8. https://doi.org/10.1016/j.jphotobiol.2010.09.002.
Yefimenko OY, Savchenko YO, Falalyeyeva TM, Beregova TV, Zholobak NM, Spivak MY, et al. Nanocrystalline cerium dioxide efficacy for gastrointestinal motility: potential for prokinetic treatment and prevention in elderly. EPMA J. 2015;6:6.
Kobyliak N, Virchenko O, Falalyeyeva T, Kondro M, Beregova T, Bodnar P, et al. Cerium dioxide nanoparticles possess anti-inflammatory properties in the conditions of the obesity-associated NAFLD in rats. Biomed Pharmacother. 2017;90:608–14. https://doi.org/10.1016/j.biopha.2017.03.099.
Babenko LP, Zholobak NM, Shcherbakov AB, Woychuk SI, Lazarenko LM. Spivak MYa: Antimicrobial action of the ceria colloid solution on opportunistic microorganisms in vitro. Mikrobiol Zh. 2012;74(3):54–62.
Engeda JC, Holliday KM, Hardy ST, Chakladar S, Lin DY, Talavera GA, et al. Transitions from ideal to intermediate cholesterol levels may vary by cholesterol metric. Sci Rep. 2018;8(1):2782. https://doi.org/10.1038/s41598-018-20660-2.
Gu Y, Wang X, Li J, Zhang Y, Zhong H, Liu R, et al. Analyses of gut microbiota and plasma bile acids enable stratification of patients for antidiabetic treatment. Nat Commun. 2017;8(1):1785. https://doi.org/10.1038/s41467-017-01682-2.
Tanaka H, Doesburg K, Iwasaki T, Mierau I. Screening of lactic acid bacteria for bile salt hydrolase activity. J Dairy Sci. 1999;82:2530–5.
Ruiz L, Margolles A, Sánchez B. Bile resistance mechanisms in Lactobacillus and Bifidobacterium. Front Microbiol. 2013;4:396. https://doi.org/10.3389/fmicb.2013.00396.
Nie YF, Hu J, Yan XH. Cross-talk between bile acids and intestinal microbiota in host metabolism and health. J Zhejiang Univ Sci B. 2015;16(6):436–46. https://doi.org/10.1631/jzus.B1400327.
Wang T, Cai G, Qiu Y, Fei N, Zhang M, Pang X, et al. Structural segregation of gut microbiota between colorectal cancer patients and healthy volunteers. ISME J. 2012;6:320–9.
Guo Z, Liu XM, Zhang QX, Shen Z, Tian FW, Zhang H, et al. Influence of consumption of probiotics on the plasma lipid profile: a meta-analysis ofrandomised controlled trials. Nutr Metab Cardiovasc Dis. 2011;21(11):844–50. https://doi.org/10.1016/j.numecd.2011.04.008.
Cho YA, Kim J. Effect of probiotics on blood lipid concentrations: a meta-analysis of randomized controlled trials. Medicine (Baltimore). 2015;94(43):e1714. https://doi.org/10.1097/MD.0000000000001714.
Ooi L-G, Liong M-T. Cholesterol-lowering effects of probiotics and prebiotics: a review of in vivo and in vitro findings. Int J Mol Sci. 2010;11(6):2499–522. https://doi.org/10.3390/ijms11062499.
Wang J, Zhang H, Chen X, Chen Y. Menghebilige, Bao Q. Selection of potential probiotic lactobacilli for cholesterol-lowering properties and their effect on cholesterol metabolism in rats fed a high-lipid diet. J Dairy Sci. 2012;95(4):1645–54.
Behrouz V, Jazayeri S, Aryaeian N, Zahedi MJ, Hosseini F. Effects of probiotic and prebiotic supplementation on leptin, adiponectin, and glycemic parameters in non-alcoholic fatty liver disease: a randomized clinical trial. Middle East J Dig Dis. 2017;9(3):150–7. https://doi.org/10.15171/mejdd.2017.66.
McPhee JB, Schertzer J. Immunometabolism of obesity and diabetes: microbiota link compartmentalized immunity in the gut to metabolic tissue inflammation. Clin Sci. 2015;129:1083–96. https://doi.org/10.1042/CS20150431.
Avishai E, Yeghiazaryan K, Golubnitschaja O. Impaired wound healing: facts and hypotheses for multi-professional considerations in predictive, preventive and personalised medicine. EPMA J. 2017;8(1):23–33. https://doi.org/10.1007/s13167-017-0081-y.
Smokovski I, Risteski M, Polivka J Jr, Zubor P, Konieczka K, Costigliola V, et al. Postmenopausal breast cancer: European challenge and innovative concepts. EPMA J. 2017;8(2):159–69. https://doi.org/10.1007/s13167-017-0094-6.
Hotamisligil GS. Inflammation and metabolic disorders. Nature. 200;444(7121):860–7 Review.
Lazarenko LM, Babenko LP, Bubnov RV, Demchenko OM, Zotsenko VM, Boyko NV, et al. Imunobiotics are the novel biotech drugs with antibacterial and immunomodulatory properties. Mikrobiol Zh. 2017;79(1):66–75.
Lazarenko L, Melnikova O, Babenko L, Bubnov R, Beregova T, Falalyeyeva T, et al. Lactobacillus and Bifidobacteria probiotic strains improve glycemic and inflammation profiles in obesity model in mice. Preprints. 2018. https://doi.org/10.20944/preprints201808.0169.v1.
Chistoserdova L. Lanthanides: New life metals? World J Microbiol Biotechnol. 2016;32(8):138. https://doi.org/10.1007/s11274-016-2088-2.
Skovran E, Martinez-Gomez NC. Microbiology. Just add lanthanides. Science. 2015;348(6237):862–3. https://doi.org/10.1126/science.aaa9091.
Cai L, Nyachoti CM, Kim IH. Impact of rare earth element-enriched yeast on growth performance, nutrient digestibility, blood profile, and fecal microflora in finishing pigs. Can J Anim Sci. 2018;98:347–53.
Oró D, Yudina T, Fernández-Varo G, Casals E, Reichenbach V, Casals G, et al. Cerium oxide nanoparticles reduce steatosis, portal hypertension and display anti-inflammatory properties in rats with liver fibrosis. J Hepatol. 2016;64(3):691–8. https://doi.org/10.1016/j.jhep.2015.10.020.
Parra-Robert M, Casals E, Massana N, Zeng M, Perramón M, Fernández-Varo G, Morales-Ruiz M, Puntes V, Jiménez W, Casals G. Beyond the scavenging of reactive oxygen species (ROS): direct effect of cerium oxide nanoparticles in reducing fatty acids content in an in vitro model of hepatocellular steatosis. Biomolecules. 2019;9(9). https://doi.org/10.3390/biom9090425.
Malekkhaiat Häffner S, Malmsten M. Membrane interactions and antimicrobial effects of inorganic nanoparticles. Adv Colloid Interf Sci. 2017;248:105–28. https://doi.org/10.1016/j.cis.2017.07.029.
Frost R, Svedhem S. Characterization of nanoparticle-lipid membrane interactions using QCM-D. Methods Mol Biol. 2013;991:127–37. https://doi.org/10.1007/978-1-62703-336-7_13.
Contini C, Schneemilch M, Gaisford S, Quirke N. Nanoparticle–membrane interactions. J Exp Nanosci. 2018;13(1):62–81. https://doi.org/10.1080/17458080.2017.1413253.
Alpaslan E, Geilich BM, Yazici H, Webster TJ. pH-controlled cerium oxide nanoparticle inhibition of both Gram-positive and Gram-negative bacteria growth. Sci Rep. 2017;7:45859. https://doi.org/10.1038/srep45859.
Pulido-Reyes G, Rodea-Palomares I, Das S, Sakthivel TS, Leganes F, Rosal R, et al. Untangling the biological effects of cerium oxide nanoparticles: the role of surface valence states. Sci Rep. 2015;5:15613.
Pelletier DA, Suresh AK, Holton GA, McKeown CK, Wang W, Gu B, et al. Effects of engineered cerium oxide nanoparticles on bacterial growth and viability. Appl Environ Microbiol. 2010;76(24):7981–9. https://doi.org/10.1128/AEM.00650-10.
Kamika I, Tekere M. Impacts of cerium oxide nanoparticles on bacterial community in activated sludge. AMB Express. 2017;7:63. https://doi.org/10.1186/s13568-017-0365-6.
Zholobak NM, Ivanov VK, Shcherbakov AB. Interaction of nanoceria with microorganisms. In: Grumezescu AM, editor. Nanobiomaterials in Antimicrobial Therapy. Bucharest: Elsevier Inc.; 2016. p. 419–50. https://doi.org/10.1016/B978-0-323-42864-4.00012-9.
Hickey MW, Hillier AJ, Jago GR. Transport and metabolism of lactose, glucose, and galactose in homofermentative lactobacilli. Appl Environ Microbiol. 1986;51(4):825–31.
Pol A, Barends TR, Dietl A, Khadem AF, Eygensteyn J, Jetten MS, et al. Rare earth metals are essential for methanotrophic life in volcanic mudpots. Environ Microbiol. 2014;16(1):255–64.
Good NM, Vu HN, Suriano CJ, Subuyuj GA, Skovran E, Martinez-Gomez NC. Pyrroloquinoline quinone ethanol dehydrogenase in methylobacterium extorquens AM1 extends lanthanide-dependent metabolism to multicarbon substrates. J Bacteriol. 2016;198(22):3109–18.
Akagawa M, Minematsu K, Shibata T, Kondo T, Ishii T, Uchida K. Identification of lactate dehydrogenase as a mammalian pyrroloquinoline quinone (PQQ)-binding protein. Sci Rep. 2016;6:26723.
Li N, Duan Y, Zhou M, Liu C, Hong F. The effects of lanthanoid on the structure–function of lactate dehydrogenase from mice heart. Biol Trace Elem Res. 2009;132(1-3):164–75.
Hirst SM, Karakoti AS, Tyler RD, Sriranganathan N, Seal S, Reilly CM. Anti-inflammatory properties of cerium oxide nanoparticles. Small. 2009;5(24):2848–56. https://doi.org/10.1002/smll.200901048.
Fu H, Yuan J, Gao H. Microbial oxidative stress response: novel insights from environmental facultative anaerobic bacteria. Arch Biochem Biophys. 2015;584:28–35. https://doi.org/10.1016/j.abb.2015.08.012 Review.
Dickson I. Anaerobic intestine-on-a-chip system enables complex microbiota co-culture. Nat Rev Gastroenterol Hepatol. 2019;16(7):390. https://doi.org/10.1038/s41575-019-0162-6.
Carvajal S, Perramón M, Oró D, Casals E, Fernández-Varo G, Casals G, et al. Cerium oxide nanoparticles display antilipogenic effect in rats with non-alcoholic fatty liver disease. Sci Rep. 2019;9(1):1–20.
Parra-Robert M, Casals E, Massana N, Zeng M, Perramón M, Fernández-Varo G, Morales-Ruiz M, Puntes V, Jiménez W, Casals G. Beyond the Scavenging of Reactive Oxygen Species (ROS): Direct Effect of Cerium Oxide Nanoparticles in Reducing Fatty Acids Content in an In Vitro Model of Hepatocellular Steatosis. Biomolecules. 2019;9(9). pii: E425. https://doi.org/10.3390/biom9090425.
Lye HS, Khoo BY, Karim AA, Rusul G, Liong MT. Ultrasound enhanced growth and cholesterol removal of Lactobacillus fermentum FTDC 1311 in the parent cells but not the subsequent passages. Ultrason Sonochem. 2012;19(4):901–8. https://doi.org/10.1016/j.ultsonch.2011.12.018.].
Lye HS, Alias KA, Rusul G, Liong MT. Ultrasound treatment enhances cholesterol removal ability of lactobacilli. Ultrason Sonochem. 2012;19(3):632–41. https://doi.org/10.1016/j.ultsonch.2011.08.004.
Lye HS, Karim AA, Rusul G, Liong MT. Electroporation enhances the ability of lactobacilli to remove cholesterol. J Dairy Sci. 2011;94(10):4820–30. https://doi.org/10.3168/jds.2011-4426.
Bubnov RV, Spivak MY. Sonoporation delivery of inorganic nanoparticles into bacterial cell of probiotic strains using diagnostic ultrasound machine. Ultrasound Med Biol. 2019;45:S83. https://doi.org/10.1016/j.ultrasmedbio.2019.07.282.
Мokrozub VV, Lazarenko LM, Sichel LM, Babenko LP, Lytvyn PM, Demchenko OM, Melnichenko YO, Boyko NV, Biavati B, DiGioia D, Bubnov RV, Spivak MY. The role of beneficial bacteria wall elasticity in regulating innate immune response. EPMA J. 2015;6(1):13. https://doi.org/10.1186/s13167-015-0035-1.
Bubnov R, Babenko L, Lazarenko L, et al. Lactobacillus and Bifidobacterium strains and compositions with nanoceria reduce cholesterol levels in an obesity mouse model. EFSA congress 2018 in Parma, Italy. EFSA Journal 09/2018; 16(Suppl. 1):157. https://www.efsa.europa.eu/sites/default/files/event/180918-conference/conference18-EFSA_Journal_abstracts.pdf. (Accessed 25.10.2019).
Bubnov R, Spivak M. Towards Individualized Use of Probiotics and Prebiotics for Metabolic Syndrome and Associated Diseases Treatment: Does Pathophysiology-Based Approach Work and Can Anticipated Evidence Be Completed?. Preprints 2018, 2018090185. https://doi.org/10.20944/preprints201809.0185.v1.
Acknowledgments
The study was conducted with the support of the State Agency on Science, Innovations and Informatisation of Ukraine.
Author information
Authors and Affiliations
Contributions
Rostyslav Bubnov created concepts and designed the study, participated in experiments, performed ultrasound investigations and analytical procedures, and drafted discussion and outlook. Lidiia Babenko and Liudmyla Lazarenko performed experiments on animals, corresponding analysis, and statistical evaluation, and created the first draft of manuscript. Maryna Kryvtsova, Nadiya Zholobak, and Oleksandr Shcherbakov participated in analytical approaches. Olga Golubnitschaja contributed to main concepts of the study, data interpretation and multidisciplinary aspects, and strengthened the expertise related to predictive, preventive, and personalized medicine. Mykola Spivak supervised the project and contributed to the data interpretation.
All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Ethics approval and consent to participate
This study has been approved by the ethics committee of institutional review board and Special Academic Council on Doctoral Thesis of D.K. Zabolotny Institute of Microbiology and Virology of the National Academy of Sciences of Ukraine (protocol N 7 issued 03.07.2018).
No human subjects were included into the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bubnov, R., Babenko, L., Lazarenko, L. et al. Can tailored nanoceria act as a prebiotic? Report on improved lipid profile and gut microbiota in obese mice. EPMA Journal 10, 317–335 (2019). https://doi.org/10.1007/s13167-019-00190-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13167-019-00190-1