Abstract
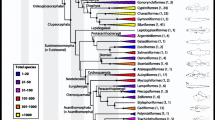
Annelida is an ecologically and morphologically diverse phylum within the Lophotrochozoa whose members occupy a wide range of environments and show diverse life styles. The phylogeny of this group comprising more than 17,000 species remained controversial for a long time. By using next-generation sequencing and phylogenomic analyses of huge data matrices, it was finally possible to reach a well-supported and resolved annelid backbone tree. Most annelid diversity is comprised in two reciprocal monophyletic groups, Sedentaria and Errantia, which are named after the predominant life style of their members. Errantia include Aciculata (Phyllodocida + Eunicida) and Protodriliformia, which is a taxon of interstitial polychaetes. Sedentaria comprise most of the polychaete families formerly classified as Canalipalpata or Scolecida, as well as the Clitellata. Six taxa branch as a basal grade outside of this major radiation: Oweniidae, Magelonidae, Chaetopteridae, Sipuncula, Amphinomida, and Lobatocerebrum. Oweniidae and Magelonidae form a monophyletic group which we name Palaeoannelida, which constitutes the sister taxon of the remaining annelids. The early splits of annelid phylogeny date back to the Cambrian. The new annelid phylogeny highlights the variability and lability of annelid body plans, and many instances of simplifications of body plan as adaptations to new life styles can be found. Therefore, annelids will be an appropriate model to understand major transitions in the evolution of Bilateria in general. Evolutionary developmental studies are one way to investigate macroevolutionary transition in annelids. We briefly summarize the state of developmental model organisms in Annelida and also propose new candidates on the background of the phylogeny.



Similar content being viewed by others
References
Achim, K., Pettit, J.-B., Saraiva, L. R., Gavriouchkina, D., Larsson, T., Arendt, D., et al. (2015). High-throughput spatial mapping of single-cell RNA-seq data to tissue of origin. Nature Biotechnology, 33, 503–509.
Aguado, M. T., Capa, M., Oceguera-Figueroa, A., & Rouse, G. W. (2014). Annelida. In P. Vargas & R. Zardoya (Eds.), The tree of life: evolution and classification of living organisms (pp. 254–269). Sunderland: Sinauer.
Aguado, M., Helm, C., Weidhase, M., & Bleidorn, C. (2015a). Description of a new syllid species as a model for evolutionary research of reproduction and regeneration in annelids. Organisms, Diversity and Evolution, 15, 1–21.
Aguado, M. T., Glasby, C. J., Schroeder, P. C., Weigert, A., & Bleidorn, C. (2015b). The making of a branching annelid: an analysis of complete mitochondrial genome and ribosomal data of Ramisyllis multicaudata. Scientific Reports, 5, 12072.
Almeida, W., Christoffersen, M., Amorim, D., Garraffoni, A., & Silva, G. (2003). Polychaeta, Annelida and Articulata are not monophyletic: articulating the Metameria (Metazoa, Coelomata). Revista Brasileira de Zoologia, 20, 23–57.
Andrade, S. C. S., Novo, M., Kawauchi, G. Y., Worsaae, K., Pleijel, F., Giribet, G., et al. (2015). Articulating “archiannelids”: phylogenomics and annelid relationships, with emphasis on meiofaunal taxa. Molecular Biology and Evolution, 32, 2860–2875.
Arenas-Mena, C. (2007). Sinistral equal-size spiral cleavage of the indirectly developing polychaete Hydroides elegans. Developmental Dynamics, 23, 1611–1622.
Arendt, D. (2011). Annelids who's who. Nature, 471, 44–45.
Arendt, D., Denes, A. S., Jékely, G., & Tessmar-Raible, K. (2008). The evolution of nervous system centralization. Philosophical Transactions of the Royal Society, B: Biological Sciences, 363, 1523–1528.
Arendt, D., Technau, U., & Wittbrodt, J. (2001). Evolution of the bilaterian larval foregut. Nature, 409, 81–85.
Arendt, D., Tessmar-Raible, K., Snyman, H., Dorresteijn, A. W., & Wittbrodt, J. (2004). Ciliary photoreceptors with a vertebrate-type opsin in an invertebrate brain. Science, 306, 869–871.
Arias, A., Barroso, R., Anadón, N., & Paiva, P. C. (2013). On the occurrence of the fireworm Eurythoe complanata complex (Annelida, Amphinomidae) in the Mediterranean Sea with an updated revision of the alien Mediterranean amphinomids. ZooKeys, 337, 19–33.
Backfisch, B., Kozin, V. V., Kirchmaier, S., Tessmar-Raible, K., & Raible, F. (2014). Tools for gene-regulatory analyses in the marine annelid Platynereis dumerilii. PLoS ONE, 9, e93076.
Backfisch, B., Veedin Rajan, V. B., Fischer, R. M., Lohs, C., Arboleda, E., Tessmar-Raible, K., et al. (2013). Stable transgenesis in the marine annelid Platynereis dumerilii sheds new light on photoreceptor evolution. Proceedings of the National Academy of Sciences, 110, 193–198.
Bannister, S., Antonova, O., Polo, A., Lohs, C., Hallay, N., Valinciute, A., et al. (2014). TALENs mediate efficient and heritable mutation of endogenous genes in the marine annelid Platynereis dumerilii. Genetics, 197, 77–89.
Barroso, R., Klautau, M., Solé-Cava, A., & Paiva, P. (2010). Eurythoe complanata (Polychaeta: Amphinomidae), the ‘cosmopolitan’ fireworm, consists of at least three cryptic species. Marine Biology, 157, 69–80.
Bartolomaeus, T. (1995). Secondary monociliarity in the Annelida: monociliated epidermal cells in larvae of Magelona mirabilis (Magelonida). Microfauna Marina, 10, 327–332.
Bergter, A., Brubacher, J. L., & Paululat, A. (2008). Muscle formation during embryogenesis of the polychaete Ophryotrocha diadema (Dorvilleidae)—new insights into annelid muscle patterns. Frontiers in Zoology, 5, 1.
Bernt, M., Bleidorn, C., Braband, A., Dambach, J., Donath, A., Fritzsch, G., et al. (2013). A comprehensive analysis of bilaterian mitochondrial genomes and phylogeny. Molecular Phylogenetics and Evolution, 69, 352–364.
Bleidorn, C. (2007). The role of character loss in phylogenetic reconstruction as exemplified for the Annelida. Journal of Zoological Systematics and Evolutionary Research, 45, 299–307.
Bleidorn, C. (2009). Annelid phylogeny—molecular analysis with emphasis on model annelids. In D. Shain (Ed.), Annelids as model systems in the biological sciences (pp. 13–29). Hoboken: Wiley.
Bleidorn, C., Eeckhaut, I., Podsiadlowski, L., Schult, N., McHugh, D., Halanych, K. M., et al. (2007). Mitochondrial genome and nuclear sequence data support Myzostomida as part of the annelid radiation. Molecular Biology and Evolution, 24, 1690–1701.
Bleidorn, C., Helm, C., Weigert, A., & Aguado, M. (2015). Annelida. In A. Wanninger (Ed.), Evolutionary developmental biology of invertebrates 2 (pp. 193–230). Vienna: Springer.
Bleidorn, C., Helm, C., Weigert, A., Eeckhaut, I., Lanterbecq, D., Struck, T. H., et al. (2014). From morphology to phylogenomics: Placing the enigmatic Myzostomida in the tree of life. In J. W. Wägele & T. Bartolomaeus (Eds.), Deep Metazoan phylogeny: the backbone of the tree of life. new insights from analyses of molecules, morphology, and theory of data analysis (pp. 161–172). Berlin: De Gruyter.
Bleidorn, C., Hill, N., Erseus, C., & Tiedemann, R. (2009a). On the role of character loss in orbiniid phylogeny (Annelida): molecules vs. morphology. Molecular Phylogenetics and Evolution, 52, 57–69.
Bleidorn, C., Podsiadlowski, L., Zhong, M., Eeckhaut, I., Hartmann, S., Halanych, K. M., et al. (2009b). On the phylogenetic position of Myzostomida: can 77 genes get it wrong? BMC Evolutionary Biology, 9, 150.
Bleidorn, C., Vogt, L., & Bartolomaeus, T. (2003a). A contribution to sedentary polychaete phylogeny using 18S rRNA sequence data. Journal of Zoological Systematics and Evolutionary Research, 41, 186–195.
Bleidorn, C., Vogt, L., & Bartolomaeus, T. (2003b). New insights into polychaete phylogeny (Annelida) inferred from 18S rDNA sequences. Molecular Phylogenetics and Evolution, 29, 279–288.
Bolker, J. A. (1995). Model systems in developmental biology. Bioessays, 17, 451–455.
Boore, J. L. (1999). Animal mitochondrial genomes. Nucleic Acids Research, 27(8), 1767–1780. doi:10.1093/nar/27.8.1767.
Boore, J. L., & Brown, W. M. (1994). Mitochondrial genomes and the phylogeny of mollusks. Nautilus, 108, 61–78.
Boore, J. L., & Brown, W. M. (2000). Mitochondrial genomes of Galathealinum, Helobdella, and Platynereis: sequence and gene arrangement comparisons indicate the Pogonophora is not a phylum and Annelida and Arthropoda are not sister taxa. Molecular Biology and Evolution, 17, 988–988.
Boore, J. L., & Staton, J. L. (2002). The mitochondrial genome of the sipunculid Phascolopsis gouldii supports its association with Annelida rather than Mollusca. Molecular Biology and Evolution, 19, 127–137.
Borda, E., Yáñez-Rivera, B., Ochoa, G. M., Kudenov, J. D., Sanchez-Ortiz, C., Schulze, A., et al. (2015). Revamping Amphinomidae (Annelida: Amphinomida), with the inclusion of Notopygos. Zoologica Scripta, 44, 324–333.
Boyle, M. J., & Rice, M. E. (2014). Sipuncula: an emerging model of spiralian development and evolution. International Journal of Developmental Biology, 58, 485–499.
Boyle, M. J., & Seaver, E. C. (2010). Expression of FoxA and GATA transcription factors correlates with regionalized gut development in two lophotrochozoan marine worms: Chaetopterus (Annelida) and Themiste lageniformis (Sipuncula). EvoDevo, 1, 2.
Brown, S., Rouse, G., Hutchings, P., & Colgan, D. (1999). Assessing the usefulness of histone H3, U2 snRNA and 28S rDNA in analyses of polychaete relationships. Australian Journal of Zoology, 47, 499–516.
Brusca, R. C., & Brusca, G. J. (2003). Invertebrates (2nd ed.). Sunderland: Sinauer.
Bubko, O. V., & Minichev, Y. S. (1972). Nervous system in Oweniidae (Polychaeta). Zoologichesky Zhurnal, 51, 1288–1299.
Capa, M., Parapar, J., & Hutchings, P. (2012). Phylogeny of Oweniidae (Polychaeta) based on morphological data and taxonomic revision of Australian fauna. Zoological Journal of the Linnean Society, 166, 236–278.
Cho, S. J., Valles, Y., Giani, V. C., Jr., Seaver, E. C., & Weisblat, D. A. (2010). Evolutionary dynamics of the wnt gene family: a lophotrochozoan perspective. Molecular Biology and Evolution, 27, 1645–1658.
Clark, R. B. (1964). Dynamics in metazoan evolution. The origin of the coelom and segments. Oxford: Clarendon.
Colgan, D. J., Hutchings, P. A., & Braune, M. (2006). A multigene framework for polychaete phylogenetic studies. Organisms Diversity and Evolution, 6, 220–235.
Dales, R.P. (1963). Annelids (Hutchinson University Library). London
De Robertis, E. M. (2008). Evo-Devo: variations on ancestral themes. Cell, 132, 185–195.
Dordel, J., Fisse, F., Purschke, G., & Struck, T. H. (2010). Phylogenetic position of Sipuncula derived from multi-gene and phylogenomic data and its implication for the evolution of segmentation. Journal of Zoological Systematics and Evolutionary Research, 48, 197–207.
Dozsa-Farxas, K., & Schlaghamersky, J. (2013). Hrabeiella periglandulata (Annelida: "Polychaeta")—do apparent differences in chaetal ultrastructure indicate the existence of several species in Europe? Acta Zoologica Academiae Scientiarum Hungaricae, 59, 143–156.
Dray, N., Tessmar-Raible, K., Le Gouar, M., Vibert, L., Christodoulou, F., Schipany, K., et al. (2010). Hedgehog signaling regulates segment formation in the annelid Platynereis. Science, 329, 339–342.
Dunn, C. W., Giribet, G., Edgecombe, G. D., & Hejnol, A. (2014). Animal phylogeny and its evolutionary implications. Annual Review of Ecology, Evolution, and Systematics, 45, 371–395.
Dunn, C. W., Hejnol, A., Matus, D. Q., Pang, K., Browne, W. E., Smith, S. A., et al. (2008). Broad phylogenomic sampling improves resolution of the animal tree of life. Nature, 452, 745–749.
Eeckhaut, I., McHugh, D., Mardulyn, P., Tiedemann, R., Monteyne, D., Jangoux, M., et al. (2000). Myzostomida: a link between trochozoans and flatworms? Proceedings of the Royal Society B, 267, 1383–1392.
Eibye-Jacobsen, D. (2004). A reevaluation of Wiwaxia and the polychaetes of the Burgess Shale. Lethaia, 37, 317–335.
Eibye-Jacobsen, D., & Vinther, J. (2012). Reconstructing the ancestral annelid. Journal of Zoological Systematics and Evolutionary Research, 50, 85–87.
Fauchald, K. (1974). Polychaete phylogeny: a problem in protostome evolution. Systematic Zoology, 23, 493–506.
Fauchald, K. (1977). In The polychaete worms. Definitions and keys to the orders, families and genera. (Vol. Science Series 28): Natural History Museum of Los Angeles County.
Fauchald, K., & Rouse, G. (1997). Polychaete systematics: past and present. Zoologica Scripta, 26, 71–138.
Fauvel, P. (1923). Polychètes errantes. Faune de France, 5, 1–488.
Fauvel, P. (1927). Polychètes Sédentaires. Addenda aux Errantes, Archiannélides, Myzostomaires. Faune de France, 16, 1–494.
Ferrier, D. E. K. (2012). Evolutionary crossroads in developmental biology: annelids. Development, 139, 2643–2653.
Ferrier, D. E. K., & Holland, P. W. H. (2001). Sipunculan ParaHox genes. Evolution & Development, 3, 263–270.
Fischer, A., & Dorresteijn, A. (2004). The polychaete Platynereis dumerilii (Annelida): a laboratory animal with spiralian cleavage, lifelong segment proliferation and a mixed benthic/pelagic life cycle. Bioessays, 26, 314–325.
Fischer, A. H. L., Henrich, T., & Arendt, D. (2010). The normal development of Platynereis dumerilii (Nereididae, Annelida). Frontiers in Zoology, 7, 31.
Frobius, A. C., Matus, D. Q., & Seaver, E. C. (2008). Genomic organization and expression demonstrate spatial and temporal Hox gene colinearity in the lophotrochozoan Capitella sp I. PLoS ONE, 3, e4004.
Fröbius, A. C., & Seaver, E. C. (2006). ParaHox gene expression in the polychaete annelid Capitella sp. I. Development Genes and Evolution, 216, 81–88.
Gardiner, S. L. (1978). Fine structure of the ciliated epidermis on the tentacles of Owenia fusiformis (Polychaeta, Oweniidae). Zoomorphologie, 91, 37–48.
Giani, V. C., Yamaguchi, E., Boyle, M. J., & Seaver, E. C. (2011). Somatic and germline expression of piwi during development and regeneration in the marine polychaete annelid Capitella teleta. Evodevo, 2, 10
Giere, O., & Erseus, C. (1998). A systematic account of the Questidae (Annelida, Polychaeta), with description of new taxa. Zoologica Scripta, 27, 345–360.
Giere, O. W., & Riser, N. W. (1981). Questidae—Polychaetes with oligochaetoid morphology and development. Zoologica Scripta, 10, 95–103.
Glasby, C., & Timm, T. (2008). Global diversity of polychaetes (Polychaeta; Annelida) in freshwater. In E. V. Balian, C. Lévêque, H. Segers, & K. Martens (Eds.), Freshwater Animal Diversity Assessment (Vol. 198, pp. 107–115, Developments in Hydrobiology). Netherlands: Springer.
Golombek, A., Tobergte, S., Nesnidal, M. P., Purschke, G., & Struck, T. H. (2013). Mitochondrial genomes to the rescue—Diurodrilidae in the myzostomid trap. Molecular Phylogenetics and Evolution, 68, 312–326.
Graff, L. v. (1877). Das Genus Myzostoma (F.S. Leuckart). Leipzig: Wilhelm Engelmann.
Grube, A. E. (1850). Die Familien der Anneliden. Archiv für Naturgeschichte Berlin, 1691, 249–364.
Halanych, K. M., Dahlgren, T. G., & McHugh, D. (2002). Unsegmented Annelids? Possible origins of four lophotrochozoan worm taxa. Integrative and Comparative Biology, 42, 678–684.
Hatschek, B. (1878). Studien über Entwicklungsgeschichte der Anneliden. Ein Beitrag zur Morphologie der Bilaterien. Arbeiten aus den Zoologischen Instituten der Universität Wien, 1, 277–404
Hausdorf, B., Helmkampf, M., Meyer, A., Witek, A., Herlyn, H., Bruchhaus, I., et al. (2007). Spiralian phylogenomics supports the resurrection of Bryozoa comprising Ectoprocta and Entoprocta. Molecular Biology and Evolution, 24, 2723–2729.
Helm, C., Adamo, H., Hourdez, S., & Bleidorn, C. (2014). An immunocytochemical window into the development of Platynereis massiliensis (Annelida, Nereididae). International Journal of Developmental Biology, 58, 613–622.
Hermans, C. O. (1969). The systematic position of the Archiannelida. Systematic Zoology, 18, 85–102.
Hessling, R., & Purschke, G. (2000). Immunohistochemical (cLSM) and ultrastructural analysis of the central nervous system and sense organs in Aeolosoma hemprichi (Annelida, Aeolosomatidae). Zoomorphology, 120, 65–78.
Hill, S. D., & Savage, R. M. (2009). Evolution, development and ecology of Capitella sp. I: A Waxing Model for Polychaete Studies. In Annelids in Modern Biology (pp. 88–115): Wiley
Hints, O., & Eriksson, M. E. (2007). Diversification and biogeography of scolecodont bearing polychaetes in the Ordovician. Palaeogeography, Palaeoclimatology, Palaeoecology, 245, 95–114.
Huang, D. Y., Chen, J. Y., Vannier, J., & Salinas, J. I. S. (2004). Early Cambrian sipunculan worms from southwest China. Proceedings of the Royal Society B, 271, 1671–1676.
Irvine, S. Q., & Martindale, M. Q. (2000). Expression patterns of anterior Hox genes in the polychaete Chaetopterus: correlation with morphological boundaries. Developmental Biology, 217, 333–351.
Irvine, S. Q., Warinner, S. A., Hunter, J. D., & Martindale, M. Q. (1997). A survey of homeobox genes in Chaetopterus variopedatus and analysis of polychaete homeodomains. Molecular Phylogenetics and Evolution, 7, 331–345.
Iwasa, J. H., Suver, D. W., & Savage, R. M. (2000). The leech hunchback protein is expressed in the epithelium and CNS but not in the segmental precursor lineages. Development Genes and Evolution, 210, 277–288.
Jenner, R. A. (2014). Macroevolution of animal body plans: is there science after the tree? BioScience, 64, 653–664.
Jenner, R. A., & Wills, M. A. (2007). The choice of model organisms in evo-devo. Nature Reviews Genetics, 8(4), 311–314.
Jennings, R. M., & Halanych, K. M. (2005). Mitochondrial genomes of Clymenella torquata (Maldanidae) and Riftia pachyptila (Siboglinidae): evidence for conserved gene order in Annelida. Molecular Biology and Evolution, 22, 210–222.
Johansson, K. E. (1937). Über Lamellisabella zachsi und ihre systematische Stellung. Zoologischer Anzeiger, 117, 23–26.
Jolly, M. T., Viard, F., Gentil, F., Thiebaut, E., & Jollivet, D. (2006). Comparative phylogeography of two coastal polychaete tubeworms in the Northeast Atlantic supports shared history and vicariant events. Molecular Ecology, 15, 1841–1855.
Kerner, P., Zelada González, F., Le Gouar, M., Ledent, V., Arendt, D., & Vervoort, M. (2006). The expression of a hunchback ortholog in the polychaete annelid Platynereis dumerilii suggests an ancestral role in mesoderm development and neurogenesis. Development Genes and Evolution, 216, 821–828.
Kerbl, A., Bekkouche, N., Sterrer, W., & Worsaae, K. (2015). Detailed reconstruction of the nervous and muscular system of Lobatocerebridae with an evaluation of its annelid affinity. BMC Evolutionary Biology, 15, 277.
Kim, C. B., Moon, S. Y., Gelder, S. R., & Kim, W. (1996). Phylogenetic relationships of annelids, molluscs, and arthropods evidenced from molecules and morphology. Journal of Molecular Evolution, 43, 207–215.
Koh, B. S., & Bhaud, M. (2001). Description of Owenia gomsoni n. sp. (Oweniidae, Annelida Polychaeta) from the Yellow Sea and evidence that Owenia fusiformis is not a cosmopolitan species. Vie et milieu, 51, 77–87.
Kojima, S. (1998). Paraphyletic status of Polychaeta suggested by phylogenetic analysis based on the amino acid sequences of elongation factor-1 alpha. Molecular Phylogenetics and Evolution, 9, 255–261.
Kristensen, R. M., & Niilonen, T. (1982). Structural studies on Diurodrilus Remane (Diurodrilidae fam. n.), with description of Diurodrilus westheidei sp. n. from the Arctic interstitial meiobenthos, W. Greenland. Zoologica Scripta, 11, 1–12.
Kristof, A., Wollesen, T., Maiorova, A. S., & Wanninger, A. (2011). Cellular and muscular growth patterns during sipunculan development. Journal of Experimental Zoology Part B, Molecular and Developmental Evolution, 316B, 227–240.
Kristof, A., Wollesen, T., & Wanninger, A. (2008). Segmental mode of neural patterning in Sipuncula. Current Biology, 18, 1129–1132.
Kudenov, J. D. (1974). The reproductive biology of Eurythoe complanata (Pallas, 1766), (Polychaeta: Amphinomidae). Tucson: University of Arizona.
Kulakova, M., Bakalenko, N., Novikova, E., Cook, C. E., Eliseeva, E., Steinmetz, P. R. H., et al. (2007). Hox gene expression in larval development of the polychaetes Nereis virens and Platynereis dumerilii (Annelida, Lophotrochozoa). Development Genes and Evolution, 217, 39–54.
Kulakova, M. A., Cook, C. E., & Andreeva, T. F. (2008). ParaHox gene expression in larval and postlarval development of the polychaete Nereis virens (Annelida, Lophotrochozoa). BMC Developmental Biology, 8, 61.
Kupriyanova, E. K., ten Hove, H. A., Sket, B., Zakšek, V., Trontelj, P., & Rouse, G. W. (2009). Evolution of the unique freshwater cave-dwelling tube worm Marifugia cavatica (Annelida: Serpulidae). Systematics and Biodiversity, 7, 389–401.
Kvist, S., & Siddall, M. E. (2013). Phylogenomics of Annelida revisited: a cladistic approach using genome-wide expressed sequence tag data mining and examining the effects of missing data. Cladistics, 29, 435–448.
Lamarck, J.-B. M. (1818). Histoire naturelle des animaux sans vertebres. Deterville/Verdiere, Paris
Laumer, C. E., Bekkouche, N., Kerbl, A., Goetz, F., Neves, R. C., Sorensen, M. V., et al. (2015). Spiralian phylogeny informs the evolution of microscopic lineages. Current Biology, 25, 2000–2006.
Lauri, A., Brunet, T., Handberg-Thorsager, M., Fischer, A. H. L., Simakov, O., Steinmetz, P. R. H., et al. (2014). Development of the annelid axochord: insights into notochord evolution. Science, 345, 1365–1368.
Li, Y., Kocot, K. M., Schander, C., Santos, S. R., Thornhill, D. J., & Halanych, K. M. (2015). Molecular Phylogenetics and Evolution, 85, 221–229.
Liu, J., Ou, Q., Han, J., Li, J., Wu, Y., Jiao, G., et al. (2015). Lower Cambrian polychaete from China sheds light on early annelid evolution. The Science of Nature, 102, 1–7.
Martindale, M. Q., & Hejnol, A. (2009). A developmental perspective: changes in the position of the blastopore during bilaterian evolution. Developmental Cell, 17, 162–174.
McCormack, J. E., Hird, S. M., Zellmer, A. J., Carstens, B. C., & Brumfield, R. T. (2013). Applications of next-generation sequencing to phylogeography and phylogenetics. Molecular Phylogenetics and Evolution, 66, 526–538.
McDougall, C., Korchagina, N., Tobin, J. L., & Ferrier, D. E. K. (2011). Annelid Distalless/Dlx duplications reveal varied post-duplication fates. BMC Evolutionary Biology, 11, 241.
McHugh, D. (1997). Molecular evidence that echiurans and pogonophorans are derived annelids. Proceedings of the National Academy of Sciences of the United States of America, 94, 8006–8009.
McHugh, D. (2000). Molecular phylogeny of the Annelida. Canadian Journal of Zoology, 78, 1873–1884.
Mehr, S., Verdes, A., DeSalle, R., Sparks, J., Pieribone, V., & Gruber, D. F. (2015). Transcriptome sequencing and annotation of the polychaete Hermodice carunculata (Annelida, Amphinomidae). BMC Genomics, 16, 445.
Meyer, N., Carrillo-Baltodano, A., Moore, R., & Seaver, E. (2015). Nervous system development in lecithotrophic larval and juvenile stages of the annelid Capitella teleta. Frontiers in Zoology, 12, 15.
Milinkovitch, M. C., & Tzika, A. (2007). Escaping the mouse trap: the selection of new Evo-Devo model species. Journal of Experimental Zoology Part B: Molecular and Developmental Evolution, 308B, 337–346.
Moon, S., Kim, C., Gelder, S., & Kim, W. (1996). Phylogenetic positions of the aberrant branchiobdellidans and aphanoneurans within the Annelida as derived from 18S ribosomal RNA gene sequences. Hydrobiologia, 324, 229–236.
Mortimer, K., & Mackie, A. S. Y. (2014). Morphology, feeding and behaviour of British Magelona (Annelida: Magelonidae), with discussions on the form and function of abdominal lateral pouches. Memoirs of Museum Victoria, 71, 177–201.
Müller, M. C. M., Berenzen, A., & Westheide, W. (2003). Experiments on anterior regeneration in Eurythoe complanata (“Polychaeta”, Amphinomidae): reconfiguration of the nervous system and its function for regeneration. Zoomorphology, 122, 95–103.
Mwinyi, A., Meyer, A., Bleidorn, C., Lieb, B., Bartolomaeus, T., & Podsiadlowski, L. (2009). Mitochondrial genome sequence and gene order of Sipunculus nudus give additional support for an inclusion of Sipuncula into Annelida. BMC Genomics, 10, 27.
Newby, W. W. (1940). The embryology of the echiuroid worm, Urechis caupo. (Vol. 16, Memoirs of the American Philosophical Society). Philadelphia: American Philosophical Society.
Oakley, T., & Speiser, D. I. (2015). How complexity originates: the evolution of animal eyes. Annual Review of Ecology, Evolution, and Systematics, 46, 237–260.
Oyama, A., & Shimizu, T. (2007). Transient occurrence of vasa-expressing cells in nongenital segments during embryonic development in the oligochaete annelid Tubifex tubifex. Development Genes and Evolution, 217, 675–690.
Parry, L., Tanner, A., & Vinther, J. (2014). The origin of annelids. Palaeontology, 57, 1091–1103.
Parry, L., Vinther, J., & Edgecombe, G. D. (2015). Cambrian stem-group annelids and a metameric origin of the annelid head. Biology Letters, 11, 20150763.
Pfeifer, K., Dorresteijn, A. W. C., & Frobius, A. C. (2012). Activation of Hox genes during caudal regeneration of the polychaete annelid Platynereis dumerilii. Development Genes and Evolution, 222, 165–179.
Prud'homme, B., de Rosa, R., Arendt, D., Julien, J. F., Pajaziti, R., Dorresteijn, A. W. C., et al. (2003). Arthropod-like expression patterns of engrailed and wingless in the annelid Platynereis dumerilii suggest a role in segment formation. Current Biology, 13, 1876–1881.
Prud'homme, B., Latillot, N., Balavoine, G., Adoutte, A., & Vervoort, M. (2002). Phylogenetic analysis of the wnt gene family: Insights from lophotrochozoan members. Current Biology, 12, 1395–1400.
Purschke, G. (1999). Terrestrial polychaetes—models for the evolution of the Clitellata (Annelida)? Hydrobiologia, 406, 87–99.
Purschke, G. (2002). On the ground pattern of Annelida. Organisms, Diversity and Evolution, 2, 181–196.
Purschke, G. (2003). Is Hrabeiella periglandulata (Annelida, "Polychaeta") the sister group of Clitellata? Evidence from an ultrastructural analysis of the dorsal pharynx in H. periglandulata and Enchytraeus minutus (Annelida, Clitellata). Zoomorphology, 122, 55–66.
Purschke, G., Bleidorn, C., & Struck, T. H. (2014). Systematics, evolution and phylogeny of Annelida—a morphological perspective. Memoirs of Museum Victoria, 71, 247–269.
Purschke, G., Hessling, R., & Westheide, W. (2000). The phylogenetic position of the Clitellata and the Echiura—on the problematic assessment of absent characters. Journal of Zoological Systematics and Evolutionary Research, 38, 165–173.
Purschke, G., & Jouin, C. (1988). Anatomy and ultrastructure of the ventral pharyngeal organs of Saccocirrus (Saccocirridae) and Protodriloides (Protodriloidae fam. n.) with remarks on the phylogenetic relationships within the Protodrilida (Annelida: Polychaeta). Journal of Zoology, 215, 405–432.
de Quatrefages, A. (1865). Note sur la classification des Annélides. Comptes rendus hebdomadaires des séances de l'Académie des sciences. Paris, 60, 586–600.
Richter, S., Schwarz, F., Hering, L., Böggemann, M., & Bleidorn, C. (2015). The utility of genome skimming for phylogenomic analyses as demonstrated for glycerid relationships (Annelida, Glyceridae). Genome Biology and Evolution, 7, 3443–3462.
Rieger, R. M. (1980). A new group of interstitial worms, Lobatocerebridae nov. fam. (Annelida) and its significance for metazoan phylogeny. Zoomorphologie, 95, 41–84.
Rieger, R. M. (1981). Fine structure of the body wall, nervous system, and digestive tract in the Lobatocerebridae Rieger and the organization of the gliointerstitial system in Annelida. Journal of Morphology, 167, 139–165.
Rieger, R. M. (1988). Comparative ultrastructure and the Lobatocerebridae: keys to understand the phylogenetic relationship of Annelida and the acoelomates. Microfauna Marina, 4, 373–382.
Rivera, A. S., & Weisblat, D. A. (2009). And Lophotrochozoa makes three: Notch/Hes signaling in annelid segmentation. Development Genes and Evolution, 219, 37–43.
Rota, E., Martin, P., & Erseus, C. (2001). Soil-dwelling polychaetes: enigmatic as ever? Some hints on their phylogenetic relationships as suggested by a maximum parsimony analysis of 18S rRNA gene sequences. Contributions to Zoology, 70, 127–138.
Rouse, G. W., & Fauchald, K. (1997). Cladistics and polychaetes. Zoologica Scripta, 26, 139–204.
Rouse, G. W., & Fauchald, K. (1998). Recent views on the status, delineation and classification of the Annelida. American Zoologist, 38, 953–964.
Rouse, G. W., & Pleijel, F. (2001). Polychaetes (Polychaetes): Oxford University Press
Rouse, G. W., & Pleijel, F. (2006). Annelid phylogeny and systematics. In G. W. Rouse & F. Pleijel (Eds.), Reproductive biology and phylogeny of Annelida (Vol. 4, pp. 3–21). Enfield: Science.
Rousset, V., Pleijel, F., Rouse, G. W., Erseus, C., & Siddall, M. E. (2007). A molecular phylogeny of annelids. Cladistics, 23, 41–63.
Rousset, V., Rouse, G. W., Siddall, M. E., Tillier, A., & Pleijel, F. (2004). The phylogenetic position of Siboglinidae (Annelida) inferred from 18S rRNA, 28S rRNA and morphological data. Cladistics, 20, 518–533.
Rudel, D., & Sommer, R. J. (2003). The evolution of developmental mechanisms. Developmental Biology, 264, 15–37.
Seaver, E. C. (2003). Segmentation: mono- or polyphyletic? The International Journal of Developmental Biology, 47, 583–595.
Seaver, E. C., & Kaneshige, L. M. (2006). Expression of ‘segmentation’ genes during larval and juvenile development in the polychaetes Capitella sp. I and H. elegans. Developmental Biology, 289, 179–194.
Seaver, E. C., Paulson, D. A., Irvine, S. Q., & Martindale, M. Q. (2001). The spatial and temporal expression of Ch-en, the engrailed gene in the polychaete Chaetopterus, does not support a role in body axis segmentation. Developmental Biology, 236, 195–209.
Seaver, E. C., & Shankland, M. (2001). Establishment of segment polarity in the ectoderm of the leech Helobdella. Development, 128, 1629–1641.
Seaver, E. C., Thamm, K., & Hill, S. D. (2005). Growth patterns during segmentation in the two polychaete annelids, Capitella sp. I and Hydroides elegans: comparisons at distinct life history stages. Evolution & Development, 7, 312–326.
Seaver, E. C., Yamaguchi, E., Richards, G. S., & Meyer, N. P. (2012). Expression of the pair-rule gene homologs runt, Pax3/7, even-skipped-1 and even-skipped-2 during larval and juvenile development of the polychaete annelid Capitella teleta does not support a role in segmentation. EvoDevo, 3, 8.
Shen, X., Ma, X. Y., Ren, J. F., & Zhao, F. Q. (2009). A close phylogenetic relationship between Sipuncula and Annelida evidenced from the complete mitochondrial genome sequence of Phascolosoma esculenta. BMC Genomics, 10, 136.
Simakov, O., Marletaz, F., Cho, S.-J., Edsinger-Gonzales, E., Havlak, P., Hellsten, U., et al. (2013). Insights into bilaterian evolution from three spiralian genomes. Nature, 493, 526–531.
Smart, T. I., & Von Dassow, G. (2009). Unusual development of the Mitraria larva in the polychaete Owenia collaris. Biological Bulletin, 217, 253–268.
Smith, P. R., Ruppert, E. E., & Gardiner, S. L. (1987). A deuterostome-like nephridium in the Mitraria larva of Owenia fusiformis (Polychaeta, Annelida). Biological Bulletin, 172(3), 315–323.
Song, M. H., Huang, F. Z., Chang, G. Y., & Weisblat, D. A. (2002). Expression and function of an even-skipped homolog in the leech Helobdella robusta. Development, 129, 3681–3692.
Sperling, E. A., Vinther, J., Moy, V. N., Wheeler, B. M., Semon, M., & Briggs, D. E. G. (2009). MicroRNAs resolve an apparent conflict between annelid systematics and their fossil record. Proceedings of the Royal Society B, 276, 4315–4322.
Struck, T., Hessling, R., & Purschke, G. (2002a). The phylogenetic position of the Aeolosomatidae and Parergodrilidae, two enigmatic oligochaete-like taxa of the 'Polychaeta', based on molecular data from 18S rDNA sequences. Journal of Zoological Systematics and Evolutionary Research, 40, 155–163.
Struck, T. H., Westheide, W., & Purschke, G. (2002b). Progenesis in Eunicida ("Polychaeta," Annelida)—separate evolutionary events? Evidence from molecular data. Molecular Phylogenetics and Evolution, 25, 190–199.
Struck, T. H. (2011). Direction of evolution within Annelida and the definition of Pleistoannelida. Journal of Zoological Systematics and Evolutionary Research, 49, 340–345.
Struck, T. H. (2013a). The impact of paralogy on phylogenomic studies—a case study on annelid relationships. PLoS ONE, 8, e62892.
Struck, T. H. (2013b). Phylogeny. In W. Westheide, & G. Purschke (Eds.), Handbook of Zoology. Annelida. Berlin: DeGruyter.
Struck, T. H., Golombek, A., Weigert, A., Franke, F. A., Westheide, W., Purschke, G., et al. (2015). The evolution of annelids reveals two adaptive routes to the interstitial realm. Current Biology, 25, 1993–1999.
Struck, T. H., Nesnidal, M. P., Purschke, G., & Halanych, K. M. (2008). Detecting possibly saturated positions in 18S and 28S sequences and their influence on phylogenetic reconstruction of Annelida (Lophotrochozoa). Molecular Phylogenetics and Evolution, 48, 628–645.
Struck, T. H., Paul, C., Hill, N., Hartmann, S., Hosel, C., Kube, M., et al. (2011). Phylogenomic analyses unravel annelid evolution. Nature, 471, 95–98.
Struck, T. H., & Purschke, G. (2005). The sister group relationship of Aeolosomatidae and Potamodrilidae (Annelida:"Polychaeta")—a molecular phylogenetic approach based on 18S rDNA and cytochrome oxidase I. Zoologischer Anzeiger, 243, 281–293.
Struck, T. H., Schult, N., Kusen, T., Hickman, E., Bleidorn, C., McHugh, D., et al. (2007). Annelid phylogeny and the status of Sipuncula and Echiura. BMC Evolutionary Biology, 7, 57.
Struck, T. H., Wey-Fabrizius, A. R., Golombek, A., Hering, L., Weigert, A., Bleidorn, C., et al. (2014). Platyzoan paraphyly based on phylogenomic data supports a noncoelomate ancestry of Spiralia. Molecular Biology and Evolution, 31, 1833–1849.
Tautz, D. (2004). Segmentation. Developmental Cell, 7, 301–312.
Telford, M. J., & Budd, G. E. (2003). The place of phylogeny and cladistics in Evo-Devo research. International Journal of Developmental Biology, 47, 479–490.
Tessmar-Raible, K., & Arendt, D. (2003). Emerging systems: between vertebrates and arthropods, the Lophotrochozoa. Current Opinion in Genetics & Development, 13, 331–340.
Tessmar-Raible, K., Steinmetz, P. R. H., Snyman, H., Hassel, M., & Arendt, D. (2005). Fluorescent two-color whole mount in situ hybridization in Platynereis dumerilii (Polychaeta, Annelida), an emerging marine molecular model for evolution and development. Biotechniques, 39, 460–464.
Wanninger, A., Koop, D., Bromham, L., Noonan, E., & Degnan, B. M. (2005). Nervous and muscle system development in Phascolion strombus (Sipuncula). Development Genes and Evolution, 215, 509–518.
Weidhase, M., Bleidorn, C.; Beckers, P., & Helm, C. (in press) Myoanatomy and anterior muscle regeneration of the fireworm Eurythoe cf. complanata (Annelida: Amphinomidae). Journal of Morphology.
Weigert, A., Golombek, A., Gerth, M., Schwarz, F., Struck, T. H., & Bleidorn, C. (2016). Evolution of mitochondrial gene order in Annelida. Molecular Phylogenetics and Evolution, 94, 196–206.
Weigert, A., Helm, C., Meyer, M., Nickel, B., Arendt, D., Hausdorf, B., et al. (2014). Illuminating the base of the annelid tree using transcriptomics. Molecular Biology and Evolution, 31, 1391–1401.
Weisblat, D. A., & Kuo, D. H. (2009). Helobdella (leech): a model for developmental studies. Cold Spring Harbor Protocols, 2009(4), pdb emo121.
Werbrock, A. H., Meiklejohn, D. A., Sainz, A., Iwasa, J. H., & Savage, R. M. (2001). A polychaete hunchback ortholog. Developmental Biology, 235, 476–488.
Westheide, W. (2008). Polychaetes: interstitial families: keys and notes for the identification of the species. Synopses of the British fauna (New Series), 44 (second edition). Field Studies Council: Shrewsbury, UK
Wilson, D. P. (1982). The larval development of three species of Magelona (Polychaeta) from localities near Plymouth. Journal of the Marine Biological Association of the United Kingdom, 62, 385–401.
Winchell, C. J., & Jacobs, D. K. (2013). Expression of the Lhx genes apterous and lim1 in an errant polychaete: implications for bilaterian appendage evolution, neural development, and muscle diversification. EvoDevo, 4, 4.
Winnepenninckx, B., Backeljau, T., & De Wachter, R. (1995). Phylogeny of protostome worms derived from 18S rRNA sequences. Molecular Biology and Evolution, 12, 641–649.
Winnepenninckx, B. M. H., Van de Peer, Y., & Backeljau, T. (1998). Metazoan relationships on the basis of 18S rRNA sequences: a few years late. American Zoologist, 38, 888–906.
Worsaae, K., & Kristensen, R. M. (2005). Evolution of interstitial Polychaeta (Annelida). Hydrobiologia, 535, 319–340.
Worsaae, K., & Rouse, G. W. (2008). Is Diurodrilus an Annelid? Journal of Morphology, 269, 1426–1455.
Wu, Z. G., Shen, X., Sun, M. A., Ren, J. F., Wang, Y. J., Huang, Y. L., et al. (2009). Phylogenetic analyses of complete mitochondrial genome of Urechis unicinctus (Echiura) support that echiurans are derived annelids. Molecular Phylogenetics and Evolution, 52, 558–562.
Zantke, J., Bannister, S., Rajan, V. B. V., Raible, F., & Tessmar-Raible, K. (2014). Genetic and genomic tools for the marine annelid Platynereis dumerilii. Genetics, 197, 19–31.
Zhang, Z.-Q. (2011). Animal biodiversity: an introduction to higher-level classification and taxonomic richness. Zootaxa, 3148, 7–12.
Zrzavý, J. (2001). Myzostomida are not annelids: molecular and morphological support for a clade of animals with anterior sperm flagella. Cladistics, 17, 170–198.
Zrzavy, J., Riha, P., Pialek, L., & Janouskovec, J. (2009). Phylogeny of Annelida (Lophotrochozoa): total-evidence analysis of morphology and six genes. BMC Evolutionary Biology, 9, 189.
Acknowledgments
We would like to thank Andreas Wanninger for editing this special issue and for inviting us to present an overview of the new annelid phylogeny. We are grateful that David Weisblat and Stephanie Bannister provided pictures of their favorite model organisms, and that Alexander Semenov provided a photograph of Chaetopterus. This study has been supported by the Deutsche Forschungsgemeinschaft (BL787/5-1).
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Special Issue. The new animal phylogeny: The first 20 years
Rights and permissions
About this article
Cite this article
Weigert, A., Bleidorn, C. Current status of annelid phylogeny. Org Divers Evol 16, 345–362 (2016). https://doi.org/10.1007/s13127-016-0265-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13127-016-0265-7