Abstract
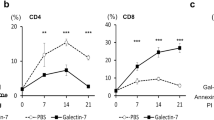
Altered glycosylation is a common feature of cancer cells and plays an important role in tumor progression. β-Galactoside α2-6-sialyltransferase 1 (ST6Gal-I) is the critical sialyltransferase responsible for the addition of α2-6-sialic acid to the terminal N-glycans on the cell surface. However, the functions and mechanism of ST6Gal-I in tumor immune escape remain poorly understood. Here, we found that ST6Gal-I overexpression promoted hepatocarcinoma cell proliferation, migration, and immune escape by increasing the levels of CD147, MMP9, MMP2, and MMP7. When CD8+ T cells were co-cultured with cell lines expressing different levels of ST6Gal-I, we found that ST6Gal-I upregulation inhibited the T cell proliferation and increased the secretion of IL-10 and TGF-β1, while secretion of IFN-γ and TNF-α was diminished. In a syngeneic tumor transplant model, ST6Gal-I upregulated Hca-P. In addition, Hepa1-6 cells formed significantly larger tumors and suppressed intratumoral penetration by CD8+ T cells. In combination, these results suggest that ST6Gal-I promotes the immune escape of hepatocarcinoma cells in the tumor microenvironment and highlight the importance of assessing ST6Gal-I status for immunotherapies.





Similar content being viewed by others
Change history
03 December 2019
Subsequently to the publication of this article, the authors have noticed that the published version of Fig. 4H contained incorrect data showing the migration of the Mock cells (left panel, Hepa1-6 cells transfected with pcDNA3.1).
References
Bresalier RS, Rockwell RW, Dahiya R, Duh QY, Kim YS (1990) Cell surface sialoprotein alterations in metastatic murine colon cancer cell lines selected in an animal model for colon cancer metastasis. Cancer Res 50:1299–1307
Bruns H, Petrulionis M, Schultze D, Al Saeedi M, Lin S, Yamanaka K, Ambrazevicius M, Strupas K, Schemmer P (2014) Glycine inhibits angiogenic signaling in human hepatocellular carcinoma cells. Amino Acids 46:969–976
Chen X, Wang L, Zhao Y, Yuan S, Wu Q, Zhu X, Niang B, Wang S, Zhang J (2016) ST6Gal-I modulates docetaxel sensitivity in human hepatocarcinoma cells via the p38 MAPK/caspase pathway. Oncotarget 7:51955–51964
Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, Luo R, Feng J, Ye S, Yang TS, Xu J, Sun Y, Liang H, Liu J, Wang J, Tak WY, Pan H, Burock K, Zou J, Voliotis D, Guan Z (2009) Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol 10:25–34
Chow MT, Moller A, Smyth MJ (2012) Inflammation and immune surveillance in cancer. Semin Cancer Biol 22:23–32
Dall’Olio F, Chiricolo M (2001) Sialyltransferases in cancer. Glycoconj J 18:841–850
Dall’Olio F, Chiricolo M, D’Errico A, Gruppioni E, Altimari A, Fiorentino M, Grigioni WF (2004) Expression of beta-galactoside alpha2,6 sialyltransferase and of alpha2,6-sialylated glycoconjugates in normal human liver, hepatocarcinoma, and cirrhosis. Glycobiology 14:39–49
Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F (2015) Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 136:E359–E386
Gross A, McDonnell JM, Korsmeyer SJ (1999) BCL-2 family members and the mitochondria in apoptosis. Genes Dev 13:1899–1911
Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144:646–674
Huang L, Xu AM, Peng Q (2015) CD147 and MMP-9 expressions in type II/III adenocarcinoma of esophagogastric junction and their clinicopathological significances. Int J Clin Exp Pathol 8:1929–1937
Kennedy KM, Dewhirst MW (2010) Tumor metabolism of lactate: the influence and therapeutic potential for MCT and CD147 regulation. Future Oncol 6:127–148
Lee JK, Capanu M, O’Reilly EM, Ma J, Chou JF, Shia J, Katz SS, Gansukh B, Reidy-Lagunes D, Segal NH, Yu KH, Chung KY, Saltz LB, Abou-Alfa GK (2013) A phase II study of gemcitabine and cisplatin plus sorafenib in patients with advanced biliary adenocarcinomas. Br J Cancer 109:915–919
Li R, Huang L, Guo H, Toole BP (2001) Basigin (murine EMMPRIN) stimulates matrix metalloproteinase production by fibroblasts. J Cell Physiol 186:371–379
Lin S, Kemmner W, Grigull S, Schlag PM (2002) Cell surface alpha 2,6 sialylation affects adhesion of breast carcinoma cells. Exp Cell Res 276:101–110
Lin S, Hoffmann K, Schemmer P (2012) Treatment of hepatocellular carcinoma: a systematic review. Liver Cancer 1:144–158
Lu J, Isaji T, Im S, Fukuda T, Hashii N, Takakura D, Kawasaki N, Gu J (2014) Beta-galactoside alpha2,6-sialyltranferase 1 promotes transforming growth factor-beta-mediated epithelial-mesenchymal transition. J Biol Chem 289:34627–34641
Mizukoshi E, Nakamoto Y, Arai K, Yamashita T, Sakai A, Sakai Y, Kagaya T, Yamashita T, Honda M, Kaneko S (2011) Comparative analysis of various tumor-associated antigen-specific t-cell responses in patients with hepatocellular carcinoma. Hepatology 53:1206–1216
Perdicchio M, Cornelissen LA, Streng-Ouwehand I, Engels S, Verstege MI, Boon L, Geerts D, van Kooyk Y, Unger WW (2016) Tumor sialylation impedes T cell mediated anti-tumor responses while promoting tumor associated-regulatory T cells. Oncotarget 7:8771–8782
Rivoltini L, Carrabba M, Huber V, Castelli C, Novellino L, Dalerba P, Mortarini R, Arancia G, Anichini A, Fais S, Parmiani G (2002) Immunity to cancer: attack and escape in T lymphocyte-tumor cell interaction. Immunol Rev 188:97–113
Schreiber RD, Old LJ, Smyth MJ (2011) Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science 331:1565–1570
Schultz MJ, Swindall AF, Bellis SL (2012) Regulation of the metastatic cell phenotype by sialylated glycans. Cancer Metastasis Rev 31:501–518
Schultz MJ, Swindall AF, Wright JW, Sztul ES, Landen CN, Bellis SL (2013) ST6Gal-I sialyltransferase confers cisplatin resistance in ovarian tumor cells. J Ovarian Res 6:25
Swindall AF, Bellis SL (2011) Sialylation of the Fas death receptor by ST6Gal-I provides protection against Fas-mediated apoptosis in colon carcinoma cells. J Biol Chem 286:22982–22990
Swindall AF, Londono-Joshi AI, Schultz MJ, Fineberg N, Buchsbaum DJ, Bellis SL (2013) ST6Gal-I protein expression is upregulated in human epithelial tumors and correlates with stem cell markers in normal tissues and colon cancer cell lines. Cancer Res 73:2368–2378
Tang W, Chang SB, Hemler ME (2004) Links between CD147 function, glycosylation, and caveolin-1. Mol Biol Cell 15:4043–4050
Thomas MB, O’Beirne JP, Furuse J, Chan AT, Abou-Alfa G, Johnson P (2008) Systemic therapy for hepatocellular carcinoma: cytotoxic chemotherapy, targeted therapy and immunotherapy. Ann Surg Oncol 15:1008–1014
Topfer K, Kempe S, Muller N, Schmitz M, Bachmann M, Cartellieri M, Schackert G, Temme A (2011) Tumor evasion from T cell surveillance. J Biomed Biotechnol 2011:918471
Wei A, Fan B, Zhao Y, Zhang H, Wang L, Yu X, Yuan Q, Yang D, Wang S (2016) ST6Gal-I overexpression facilitates prostate cancer progression via the PI3K/Akt/GSK-3beta/beta-catenin signaling pathway. Oncotarget 7:65374–65388
Wiedmann MW, Mossner J (2010) Molecular targeted therapy of biliary tract cancer--results of the first clinical studies. Curr Drug Targets 11:834–850
Yu S, Zhang L, Li N, Fan J, Liu L, Zhang J, Wang S (2012) Caveolin-1 up-regulates ST6Gal-I to promote the adhesive capability of mouse hepatocarcinoma cells to fibronectin via FAK-mediated adhesion signaling. Biochem Biophys Res Commun 427:506–512
Yuan CH, Sun XM, Zhu CL, Liu SP, Wu L, Chen H, Feng MH, Wu K, Wang FB (2015) Amphiregulin activates regulatory T lymphocytes and suppresses CD8+ T cell-mediated anti-tumor response in hepatocellular carcinoma cells. Oncotarget 6:32138–32153
Funding
This research was supported by grants from the National Natural Science Foundation of China (No.31470799 and No.31570802), the Natural Science Foundation of Liaoning Province (No. 20170540288), and the Special Fund of Dalian city for Distinguished Young Scholars (2017RJ07).
Author information
Authors and Affiliations
Contributions
Wang L. conceived and designed the study. Wang L., Li S., Yu X., Han Y., Wu Y., Wang S., and Chen X. performed the experiments. Wang L. and Li S. wrote the paper. Zhang J. and Wang S. reviewed and edited the manuscript. All authors read and approved the manuscript.
Corresponding authors
Ethics declarations
The study protocol conformed to the principles of the Declaration of Helsinki and was approved by the Ethics Committee of The First Affiliated Hospital of Dalian Medical University, Dalian City, P.R. China
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, L., Li, S., Yu, X. et al. α2,6-Sialylation promotes immune escape in hepatocarcinoma cells by regulating T cell functions and CD147/MMP signaling. J Physiol Biochem 75, 199–207 (2019). https://doi.org/10.1007/s13105-019-00674-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13105-019-00674-8