Abstract
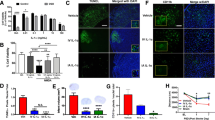
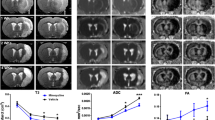
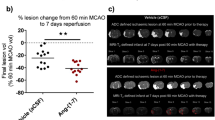
Minocycline and candesartan have both shown promise as candidate therapeutics in ischemic stroke, with multiple, and somewhat contrasting, molecular mechanisms. Minocycline is an anti-inflammatory, antioxidant, and anti-apoptotic agent and a known inhibitor of matrix metalloproteinases (MMPs). Yet, minocycline exerts antiangiogenic effects both in vivo and in vitro. Candesartan promotes angiogenesis and activates MMPs. Aligning these therapies with the dynamic processes of injury and repair after ischemia is likely to improve success of treatment. In this study, we hypothesize that opposing actions of minocycline and candesartan on angiogenesis, when administered simultaneously, will reduce the benefit of candesartan treatment. Therefore, we propose a sequential combination treatment regimen to yield a better outcome and preserve the proangiogenic potential of candesartan. In vitro angiogenesis was assessed using human brain endothelial cells. In vivo, Wistar rats subjected to 90-min middle cerebral artery occlusion (MCAO) were randomized into four groups: saline, candesartan, minocycline, and sequential combination of minocycline and candesartan. Neurobehavioral tests were performed 1, 3, 7, and 14 days after stroke. Brain tissue was collected on day 14 for assessment of infarct size and vascular density. Minocycline, when added simultaneously, decreased the proangiogenic effect of candesartan treatment in vitro. Sequential treatment, however, preserved the proangiogenic potential of candesartan both in vivo and in vitro, improved neurobehavioral outcome, and reduced infarct size. Sequential combination therapy with minocycline and candesartan improves long-term recovery and maintains candesartan’s proangiogenic potential.







Similar content being viewed by others
Abbreviations
- ARBs:
-
Angiotensin II type-1 receptor blockers
- hCMECs:
-
Human cerebral microvascular endothelial cells
- MCAO:
-
Middle cerebral artery occlusion
- MMPs:
-
Matrix metalloproteinases
- VEGF:
-
Vascular endothelial growth factor
References
Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, et al. Heart disease and stroke statistics–2014 update: a report from the American Heart Association. Circulation. 2014;129(3):e28–292. doi:10.1161/01.cir.0000441139.02102.80.
Ramaiah SS, Yan B. Low-dose tissue plasminogen activator and standard-dose tissue plasminogen activator in acute ischemic stroke in Asian populations: a review. Cerebrovasc Dis. 2013;36(3):161–6. doi:10.1159/000354162.
Ishrat T, Soliman S, Guan W, Saler M, Fagan SC. Vascular protection to increase the safety of tissue plasminogen activator for stroke. Curr Pharm Des. 2012;18(25):3677–84.
Navaratna D, Guo S, Arai K, Lo EH. Mechanisms and targets for angiogenic therapy after stroke. Cell Adhes Migr. 2009;3(2):216–23.
Chopp M, Zhang ZG, Jiang Q. Neurogenesis, angiogenesis, and MRI indices of functional recovery from stroke. Stroke J Cereb Circ. 2007;38(2 Suppl):827–31. doi:10.1161/01.STR.0000250235.80253.e9.
Xiong Y, Mahmood A, Chopp M. Angiogenesis, neurogenesis and brain recovery of function following injury. Curr Opin Investig Drugs. 2010;11(3):298–308.
Hayashi T, Noshita N, Sugawara T, Chan PH. Temporal profile of angiogenesis and expression of related genes in the brain after ischemia. J Cereb Blood Flow Metab. 2003;23(2):166–80.
Krupinski J, Kaluza J, Kumar P, Kumar S, Wang JM. Some remarks on the growth-rate and angiogenesis of microvessels in ischemic stroke. Morphometric and immunocytochemical studies. Patol Pol. 1993;44(4):203–9.
Krupinski J, Kaluza J, Kumar P, Kumar S, Wang JM. Role of angiogenesis in patients with cerebral ischemic stroke. Stroke J Cereb Circ. 1994;25(9):1794–8.
Brumm AJ, Carmichael ST. Not just a rush of blood to the head. Nat Med. 2012;18(11):1609–10. doi:10.1038/nm.2990.
Manoonkitiwongsa PS, Jackson-Friedman C, McMillan PJ, Schultz RL, Lyden PD. Angiogenesis after stroke is correlated with increased numbers of macrophages: the clean-up hypothesis. J Cereb Blood Flow Metab. 2001;21(10):1223–31. doi:10.1097/00004647-200110000-00011.
Ohab JJ, Fleming S, Blesch A, Carmichael ST. A neurovascular niche for neurogenesis after stroke. J Neurosci. 2006;26(50):13007–16. doi:10.1523/JNEUROSCI.4323-06.2006.
Simao F, Pagnussat AS, Seo JH, Navaratna D, Leung W, Lok J, et al. Pro-angiogenic effects of resveratrol in brain endothelial cells: nitric oxide-mediated regulation of vascular endothelial growth factor and metalloproteinases. J Cereb Blood Flow Metab. 2012;32(5):884–95. doi:10.1038/jcbfm.2012.2.
Guo S, Arai K, Stins MF, Chuang DM, Lo EH. Lithium upregulates vascular endothelial growth factor in brain endothelial cells and astrocytes. Stroke J Cereb Circ. 2009;40(2):652–5. doi:10.1161/STROKEAHA.108.524504.
Wang L, Zhang Z, Wang Y, Zhang R, Chopp M. Treatment of stroke with erythropoietin enhances neurogenesis and angiogenesis and improves neurological function in rats. Stroke J Cereb Circ. 2004;35(7):1732–7. doi:10.1161/01.STR.0000132196.49028.a4.
Fagan SC, Hess DC, Hohnadel EJ, Pollock DM, Ergul A. Targets for vascular protection after acute ischemic stroke. Stroke J Cereb Circ. 2004;35(9):2220–5. doi:10.1161/01.STR.0000138023.60272.9e.
Hermann DM, Zechariah A. Implications of vascular endothelial growth factor for postischemic neurovascular remodeling. J Cereb Blood Flow Metab. 2009;29(10):1620–43. doi:10.1038/jcbfm.2009.100.
Westhoff MA, Faham N, Marx D, Nonnenmacher L, Jennewein C, Enzenmuller S, et al. Sequential dosing in chemosensitization: targeting the PI3K/Akt/mTOR pathway in neuroblastoma. PLoS One. 2013;8(12):e83128. doi:10.1371/journal.pone.0083128.
De Francesco V, Hassan C, Ridola L, Giorgio F, Ierardi E, Zullo A. Sequential, concomitant and hybrid first-line therapies for H. pylori eradication: a prospective, randomized study. J Med Microbiol. 2014. doi:10.1099/jmm.0.072322-0.
Liao TV, Forehand CC, Hess DC, Fagan SC. Minocycline repurposing in critical illness: focus on stroke. Curr Top Med Chem. 2013;13(18):2283–90.
Tamargo RJ, Bok RA, Brem H. Angiogenesis inhibition by minocycline. Cancer Res. 1991;51(2):672–5.
Gilbertson-Beadling S, Powers EA, Stamp-Cole M, Scott PS, Wallace TL, Copeland J, et al. The tetracycline analogs minocycline and doxycycline inhibit angiogenesis in vitro by a non-metalloproteinase-dependent mechanism. Cancer Chemother Pharmacol. 1995;36(5):418–24.
Yao JS, Chen Y, Zhai W, Xu K, Young WL, Yang GY. Minocycline exerts multiple inhibitory effects on vascular endothelial growth factor-induced smooth muscle cell migration: the role of ERK1/2, PI3K, and matrix metalloproteinases. Circ Res. 2004;95(4):364–71. doi:10.1161/01.RES.0000138581.04174.2f.
Jung HJ, Seo I, Jha BK, Suh SI, Suh MH, Baek WK. Minocycline inhibits angiogenesis in vitro through the translational suppression of HIF-1alpha. Arch Biochem Biophys. 2014;545C:74–82. doi:10.1016/j.abb.2013.12.023.
He J, Zhang Y, Xu T, Zhao Q, Wang D, Chen CS, et al. Effects of immediate blood pressure reduction on death and major disability in patients with acute ischemic stroke: the CATIS randomized clinical trial. JAMA J Am Med Assoc. 2014;311(5):479–89. doi:10.1001/jama.2013.282543.
Engelhorn T, Goerike S, Doerfler A, Okorn C, Forsting M, Heusch G, et al. The angiotensin II type 1-receptor blocker candesartan increases cerebral blood flow, reduces infarct size, and improves neurologic outcome after transient cerebral ischemia in rats. J Cereb Blood Flow Metab. 2004;24(4):467–74. doi:10.1097/00004647-200404000-00012.
Brdon J, Kaiser S, Hagemann F, Zhao Y, Culman J, Gohlke P. Comparison between early and delayed systemic treatment with candesartan of rats after ischaemic stroke. J Hypertens. 2007;25(1):187–96. doi:10.1097/01.hjh.0000254376.80864.d3.
Guan W, Somanath PR, Kozak A, Goc A, El-Remessy AB, Ergul A, et al. Vascular protection by angiotensin receptor antagonism involves differential VEGF expression in both hemispheres after experimental stroke. PLoS One. 2011;6(9):e24551. doi:10.1371/journal.pone.0024551.
Kozak A, Ergul A, El-Remessy AB, Johnson MH, Machado LS, Elewa HF, et al. Candesartan augments ischemia-induced proangiogenic state and results in sustained improvement after stroke. Stroke J Cereb Circ. 2009;40(5):1870–6. doi:10.1161/STROKEAHA.108.537225.
Sandset EC, Bath PM, Boysen G, Jatuzis D, Korv J, Luders S, et al. The angiotensin-receptor blocker candesartan for treatment of acute stroke (SCAST): a randomised, placebo-controlled, double-blind trial. Lancet. 2011;377(9767):741–50. doi:10.1016/S0140-6736(11)60104-9.
Jauch EC, Saver JL, Adams Jr HP, Bruno A, Connors JJ, Demaerschalk BM, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke J Cereb Circ. 2013;44(3):870–947. doi:10.1161/STR.0b013e318284056a.
Saivin S, Houin G. Clinical pharmacokinetics of doxycycline and minocycline. Clin Pharmacokinet. 1988;15(6):355–66. doi:10.2165/00003088-198815060-00001.
Soliman S, El-Remessy A, Ishrat T, Pillai A, Somanath P, Ergul A, et al. Candesartan induces a prolonged proangiogenic effect and augments endothelium-mediated neuroprotection after oxygen and glucose deprivation: role of VEGF-A and B. J Pharmacol Exp Ther. 2014. doi:10.1124/jpet.113.212613.
Matsukawa N, Yasuhara T, Hara K, Xu L, Maki M, Yu G, et al. Therapeutic targets and limits of minocycline neuroprotection in experimental ischemic stroke. BMC Neurosci. 2009;10:126. doi:10.1186/1471-2202-10-126.
Ishrat T, Pillai B, Soliman S, Fouda AY, Kozak A, Johnson MH, et al. Low-dose candesartan enhances molecular mediators of neuroplasticity and subsequent functional recovery after ischemic stroke in rats. Mol Neurobiol. 2014. doi:10.1007/s12035-014-8830-6.
Bederson JB, Pitts LH, Tsuji M, Nishimura MC, Davis RL, Bartkowski H. Rat middle cerebral artery occlusion: evaluation of the model and development of a neurologic examination. Stroke J Cereb Circ. 1986;17(3):472–6.
Watanabe T, Okuda Y, Nonoguchi N, Zhao MZ, Kajimoto Y, Furutama D, et al. Postischemic intraventricular administration of FGF-2 expressing adenoviral vectors improves neurologic outcome and reduces infarct volume after transient focal cerebral ischemia in rats. J Cereb Blood Flow Metab. 2004;24(11):1205–13. doi:10.1097/01.WCB.0000136525.75839.41.
Chen J, Li Y, Wang L, Zhang Z, Lu D, Lu M, et al. Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke J Cereb Circ. 2001;32(4):1005–11.
Machado LS, Kozak A, Ergul A, Hess DC, Borlongan CV, Fagan SC. Delayed minocycline inhibits ischemia-activated matrix metalloproteinases 2 and 9 after experimental stroke. BMC Neurosci. 2006;7:56. doi:10.1186/1471-2202-7-56.
Alhusban A, Kozak A, Ergul A, Fagan SC. AT1 receptor antagonism is proangiogenic in the brain: BDNF a novel mediator. J Pharmacol Exp Ther. 2013;344(2):348–59. doi:10.1124/jpet.112.197483.
Garrido-Mesa N, Zarzuelo A, Galvez J. What is behind the non-antibiotic properties of minocycline? Pharmacol Res. 2013;67(1):18–30. doi:10.1016/j.phrs.2012.10.006.
Xu L, Fagan SC, Waller JL, Edwards D, Borlongan CV, Zheng J, et al. Low dose intravenous minocycline is neuroprotective after middle cerebral artery occlusion-reperfusion in rats. BMC Neurol. 2004;4:7. doi:10.1186/1471-2377-4-7.
Wang CX, Yang T, Shuaib A. Effects of minocycline alone and in combination with mild hypothermia in embolic stroke. Brain Res. 2003;963(1–2):327–9.
Wu J, Yang S, Hua Y, Liu W, Keep RF, Xi G. Minocycline attenuates brain edema, brain atrophy and neurological deficits after intracerebral hemorrhage. Acta Neurochir Suppl. 2010;106:147–50. doi:10.1007/978-3-211-98811-4_26.
Forder JP, Munzenmaier DH, Greene AS. Angiogenic protection from focal ischemia with angiotensin II type 1 receptor blockade in the rat. Am J Physiol Heart Circ Physiol. 2005;288(4):H1989–96. doi:10.1152/ajpheart.00839.2004.
Munzenmaier DH, Greene AS. Chronic angiotensin II AT1 receptor blockade increases cerebral cortical microvessel density. Am J Physiol Heart Circ Physiol. 2006;290(2):H512–6. doi:10.1152/ajpheart.01136.2004.
Lee S, Jilani SM, Nikolova GV, Carpizo D, Iruela-Arispe ML. Processing of VEGF-A by matrix metalloproteinases regulates bioavailability and vascular patterning in tumors. J Cell Biol. 2005;169(4):681–91. doi:10.1083/jcb.200409115.
Ebrahem Q, Chaurasia SS, Vasanji A, Qi JH, Klenotic PA, Cutler A, et al. Cross-talk between vascular endothelial growth factor and matrix metalloproteinases in the induction of neovascularization in vivo. Am J Pathol. 2010;176(1):496–503. doi:10.2353/ajpath.2010.080642.
Hollborn M, Stathopoulos C, Steffen A, Wiedemann P, Kohen L, Bringmann A. Positive feedback regulation between MMP-9 and VEGF in human RPE cells. Invest Ophthalmol Vis Sci. 2007;48(9):4360–7. doi:10.1167/iovs.06-1234.
Chetty C, Lakka SS, Bhoopathi P, Rao JS. MMP-2 alters VEGF expression via alphaVbeta3 integrin-mediated PI3K/AKT signaling in A549 lung cancer cells. Int J Cancer J Int Cancer. 2010;127(5):1081–95. doi:10.1002/ijc.25134.
Jin R, Yang G, Li G. Molecular insights and therapeutic targets for blood–brain barrier disruption in ischemic stroke: critical role of matrix metalloproteinases and tissue-type plasminogen activator. Neurobiol Dis. 2010;38(3):376–85. doi:10.1016/j.nbd.2010.03.008.
Zhao BQ, Wang S, Kim HY, Storrie H, Rosen BR, Mooney DJ, et al. Role of matrix metalloproteinases in delayed cortical responses after stroke. Nat Med. 2006;12(4):441–5. doi:10.1038/nm1387.
Yang YR, Salayandia VM, Estrata EY, Thompson JF, Rosenberg GA, Yang Y. Abstract W MP42: early treatment with minocycline promotes neurovascular remodeling of tight junctions facilitating recovery after stroke in rat brain. Stroke J Cereb Circ. 2014;45 Suppl 1:AWMP42.
Compliance with Ethical Standards
Funding Source
This study was funded by the National Institute of Health (R01-NS063965) and Veterans Affairs Merit Award (BX000891) to SCF and the American Heart Association-Southeast Affiliate (12PRE12030197) to SS.
Conflict of Interest
Authors declare no conflict of interest.
Ethical Approval
All applicable institutional guidelines for the care and use of animals were followed. All experimental protocols were approved by the Care of Experimental Animal Committee of Georgia Regents University/Institutional Animal Care and Use Committee (IACUC) of the Veterans Affairs Medical Center. This article does not contain any studies with human participants performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Soliman, S., Ishrat, T., Fouda, A.Y. et al. Sequential Therapy with Minocycline and Candesartan Improves Long-Term Recovery After Experimental Stroke. Transl. Stroke Res. 6, 309–322 (2015). https://doi.org/10.1007/s12975-015-0408-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12975-015-0408-8