Abstract
Background
Preoperative immunonutrition (IN) reduces the incidence of postoperative complications in malnourished patients undergoing upper gastrointestinal surgery. However, its effect in norm-nourished patients remains unclear. Furthermore, patients with gastric cancer undergoing laparoscopic total gastrectomy (LTG) are not routinely included in protocols of enhanced recovery after surgery (ERAS).
Objective
The aim of this study was to investigate the effects of perioperative IN in patients undergoing laparoscopic total gastrectomy (LTG) within an established ERAS pathway.
Methods
A comparative retrospective study of patients undergoing LTG, receiving an immune-enhancing feed plus maltodextrin load the day of surgery (Group A) versus patients who had the same operation but no IN nor fast track management (group B).
Results
There were no significant differences in patient demographic characteristics between the two groups but the medium age of patients in group A was older. Thirty-days postoperative complications were respectively 8.7% in Group A and 33.3% in Group B (p 0.04). Mean and median LOS for Group A and B were also significantly different: 7.2 ± 4.4 vs 10.3 ± 5.4 and 7 vs 10 days respectively.
Conclusion
Preoperative IN associated with ERAS protocol in normo-nourished patient undergoing LTG seems to reduce postoperative complications. Reduction in LOS is possibly associated to the ERAS protocol.
Clinical trial registration Clinical trials.gov: NCT05259488
Similar content being viewed by others
1 Introduction
Gastric cancer is the third leading cause of cancer related mortality worldwide. Treatment is multidisciplinary with surgical resection being the only potentially curative treatment [1]. Post-surgical complications are still high and mortality rate is up to 4%, despite advancements in surgical technique and perioperative care [2].
Enhanced recovery after surgery (ERAS) protocols, reported for gastric surgery in 2014, have proven valuable in decreasing surgical stress, improving overall outcomes and reducing the length of stay (LOS) [3]. Evidence-based guidelines focus on preoperative counselling, optimization of nutritional status and standardization of analgesia with reduction opioids and IV fluids. Laparoscopic surgery is desirable and so is early ambulation and discharge. The aim of ERAS perioperative care is the reduction of body’s physiological catabolic response to surgical stress, mediated by various stress hormones and inflammatory cytokines [4, 5] (Fig. 1).
In this context, perioperative immunonutrition (IN) is able to modulate the systemic inflammatory response by improving nutritional status and by influencing host immune response. IN is reported to decrease the incidence of postoperative infectious complications and LOS after major gastrointestinal surgery [6, 7]. However, although perioperative IN seems to reduce the rate of infective complications in malnourished patients undergoing gastrectomy, present evidences are insufficient to support a routinely use in normo-nourished patients [3, 8, 9].
The primary end-point of this study, was to compare IN within an ERAS program vs standard care in norm-nourished patients undergoing laparoscopic total gastrectomy (LTG) for advanced gastric cancer in a single unit. As secondary end-points, differences in LOS and postoperative outcomes were evaluated.
2 Material and methods
2.1 Study design and protocol
A single-center retrospective, non-parallel, comparative study.
All consecutive patients eligible for LTG for gastric cancer between November 2018 and December 2021 who meet the inclusion criteria were enrolled in the study protocol and treated with 5 days of an immune-enhancing feed plus maltodextrin load the day of surgery (Group A).
Patients and outcomes for Group A were compared with an historical control group consisting of patients undergoing LTG between January 2014 and December 2017 and treated following standard perioperative care. Data were registered on a prospectively maintained database, recording continuous and discrete variables regarding biometric data, patient-related risk factors, preoperative blood tests, tumor characteristics, neoadjuvant therapy and postoperative outcomes.
All patients had the same diagnostic work-up (endoscopy with biopsy, computed tomography, staging laparoscopy), were staged according to 8th edition of AJCC staging system and discussed in a multidisciplinary setting [10]. Neoadjuvant chemotherapy with MAGIC or FLOT regimen was given to all patients with positive lymph nodes or in case of T3/T4 N0 patients with ECOG 0–1.
Nutritional status was evaluated using the Mini Nutritional Assessment-Short form (MNA®-SF) [11]. Patients with MNA-SF score > 12 were considered normo-nourished.
IN consisted of five days of enteral nutrition with arginine + nucleotides + ω-3 fatty acids before surgery for a total of 25–30 kcal/kg/daily. Water-diluted maltodextrins (50 to 100 gr) were given two to four hours before surgery. Patients from control group did not receive nutritional support.
All patients in the two groups underwent a total laparoscopic procedure with five port technique, D2 dissection and Roux-en-Y reconstruction with side-to-side anastomoses.
All Patients in group A were treated according to ERAS 2014 guidelines [3].
Any complication (intended as any adverse event occurring during the 30-days postoperative period) including anastomotic leak, abdominal collection, bleeding, pulmonary complications (clinical symptoms, confirmed by radiological examination), surgical site infections (SSI, defined according to the Centre for Disease Control and Prevention, CDC/NHNS), was recorded and graded according to Clavien-Dindo classification [12, 13].
2.2 Inclusion and exclusion criteria
All patients aged above 18 years with a diagnosis of stage I-III gastric cancer eligible for laparoscopic D2 total gastrectomy. Exclusion criteria were acquired or congenital immunodeficiency, previous gastric surgery, malnutrition (MNA-SF < 12), preoperative infections, ASA score > 3, emergency setting, intraoperative evidence of paraaortic node involvement, distant metastasis or peritoneal spread, conversion to open surgery.
Inclusion and exclusion criteria are summarized in Table 1.
2.3 Outcomes
Study primary outcome was to evaluate 30-days postoperative complications amongst the two groups.
Secondary endpoints were: LOS and prolonged LOS (defined as any LOS greater 1.5 times the median LOS in matched cohorts), time to tolerated fluid and food intake, time to first defecation, rate of anastomotic leak, abdominal collection, bleeding, strictures, pulmonary complications, surgical site infections, 30-days reoperation, 30-days readmission, 30-days mortality.
2.4 Statistical analysis
Characteristics were summarized by means of the levels for categorical variables or by means of quantiles for continuous variables. Non-parametric tests were performed for comparisons between groups (Chi-Squared and Fisher Exact test in case of categorical variables, Wilcoxon test in case of continuous variables). Cox-Stuart test was used to test whether the data have an increasing or decreasing trend. All tests were 2-sided, accepting p < 0.05 as indicating a statistically significant difference and confidence intervals were calculated at 95% level. The analysis was performed using the R software (R Core Team (2020). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL: https://www.R-project.org/.
2.5 Ethics
This study was conducted according to the international ethical recommendations on clinical research established by the Helsinki Declaration. The study was conducted in accordance with STROBE criteria (htpp://strobe-statement.org) and registered under clinical trials.gov: NCT05259488 [14].
3 Results
3.1 Study population
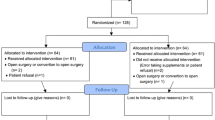
From January 2014 to December 2021 fifty-one consecutive patients diagnosed with stage I-III gastric cancer were scheduled for LTG.
Seven patients did not meet the inclusion criteria and were excluded from the analysis: Two patients were converted to open surgery and in two cases the procedure was abandoned for intraoperative evidence of peritoneal carcinosis for which other procedures can be advisable14. Three patients were excluded because of pre-operative malnutrition (MNA-SF < 12) and underwent 15 days of IV nutrition before LTG.
Forty-four patients undergoing LTG were subsequently included in the study analysis: 23 patients receiving IN (Group A) were compared to 21 patients operated between January 2014 and December 2017 that had followed standard dietary advice.
Patient selection is summarized in Fig. 2.
3.2 Baseline characteristics
Baselines patients’ characteristics are summarized in Table 2. The two groups were comparable with respect to sex, BMI, ASA score, comorbidities, preoperative albumin, MNA-SF score, staging, neoadjuvant chemotherapy. Patient’s from group A were older than patients from group B (p < 0.406).
3.3 Outcomes
Thirty-day postoperative complications were respectively 8.7% in Group A and 33.3% in Group B (p 0.04).
Mean and median LOS for Group A and B were also significantly different: 7.2 ± 4.4 vs 10.3 ± 5.4 and 7 vs 10 days respectively.
No differences were found between groups in first time to defecation, time to tolerated fluids and food intake, PLOS (any LOS greater than 13 days), anastomotic leaks, abdominal collection, strictures, bleeding, pulmonary complication, SSI, reoperation, readmission and mortality rate.
Postoperative complications occurred in two patients in Group A and seven patients in Group B.
In Group A: one patient had an anastomotic leak and sub-phrenic collection furtherly complicated by pneumonia; treatment consisted of radiological drainage, fasting and IV antibiotics (CD3a). The second patient had a postoperative bleeding and a subsequent collection requiring red cells transfusions and antibiotics (CD2).
In Group B: one patient had an anastomosis leak with a large perigastric collection and bilateral pleural effusion treated with endoscopic stent insertion and radiological drain placement. She developed a second leak at the jejunal-jejunal anastomosis, was taken back to theatre and eventually died of sepsis in ICU (CD5). The second anastomotic leak in group B, was successfully treated with endoscopic stent placement and did not have further complications (CD3a); one bleeding followed by perigastric collection required blood transfusions and IV antibiotics (CD3a), whilst a postoperative intraluminal bleeding was treated with transfusions only (CD2). One pancreatic fistula, complicated by left-sided pneumonia was also treated conservatively with antibiotics (CD2) Two patients in group B were readmitted: one for SSI on specimen extraction site requiring vaacum assisted therapy (CD2) and one because of a subfrenic collection requiring antibiotics and radiological drainage (CD3a).
Results of primary and secondary outcomes are summarized in Table 3.
4 Discussion
Application of evidence-based perioperative care protocols seems effective in ameliorate outcomes of colorectal, hepatobiliary and upper gastrointestinal surgery [16,17,18,19]. Enhanced recovery after surgery (ERAS) has progressed especially in colorectal surgery, but current guidelines point to the lack of evidence in upper gastrointestinal surgery. Since 2017, four meta-analysis have been published evaluating the state of the ERAS protocols in gastric cancer surgery [20,21,22,23].
Two papers focused on postoperative outcomes, demonstrating rapid recovery and shorter postoperative stay after laparoscopic gastrectomy [20, 21]. Ding et al. highlighted an improvement of postoperative inflammatory response but increased readmission rate without differences in postoperative complications [20]. An increased readmission rate was also found by Lee et al. and Wee et al., even if LOS, complication rates, return to bowel functions, were lower [24, 25]. Wang et al., in their metanalysis assessed the safety and efficacy of ERAS that led to a reduction of surgical stress and convalescence, and to an improvement of nutritional status [23]. Almost all papers suggested that ERAS is effective in gastric cancer surgery helping reducing costs, shortening LOS and complication rate especially when associated to laparoscopic surgery. However, in particular laparoscopic total gastrectomy (LTG) still represent a great challenge and it is not fully accepted in the common clinical practice even though, in experienced hands, is safe and feasible providing good results [26].
In most protocols of ERAS, large space is dedicated to perioperative nutrition [27,28,29,30]. Malnutrition affects negatively the host immune response and is associated with increased morbidity after surgery [31,32,33,34,35]. However, results from studies on perioperative IN are inconsistent and, although a benefit cannot be excluded, a clear evidence is still lacking, especially in norm-nourished patients [6, 27, 33].
IN with arginine, omega 3 fatty acids, nucleotides, glutamine, etc., given in the perioperative period, could reduce the synthesis of pro-inflammatory cytokines, while it could stimulate the production of glutathione, which can decrease oxidative injury. Arginine is a key element in wound and anastomosis healing [36], omega-3 and 6 play an anti-inflammatory role by contrasting oxidative injury, downregulating arachidonic acid. The addition of carbohydrates loading prevents the insulin-resistance due to cortisol and glucagon raised after surgery [37].
Our study focused on preoperative IN in a homogenous group of normo-nourished gastric cancer patients undergoing LTG following an ERAS protocol [38,39,40,41,42]. Weight loss is particularly important for patients with gastric cancer undergoing total gastrectomy and every effort is made to reduce the risk of perioperative malnutrition. Only few reports of IN in patients undergoing LTG have been published [26, 43,44,45].
To assess patients’ nutritional status, it was used the MNA-SF questionnaire, a 6-questions based malnutrition screening test, capable to individualize malnourished patients but also patients at high-risk of malnutrition.
The rate of severe complication was lower in the group of patients who had preoperative IN compared to the control group (8.7% vs 33.3%) and lower was also the LOS (7.2 vs 10.3 days). Patients in the study group were older than in the control; it is reported that age is a potential conflicting variable to the efficacy of ERAS protocol but in this single centre experience, the level of compliance was high [46, 47]. The fact that all patients in study group were treated accordingly to one single ERAS protocol, implemented with IN and followed up in a single unit, is one of the strengths of this study. A single surgeon has performed all the surgical procedures and this possibly reduce the risk of technical bias. The major limitation of this retrospective study is that is prone to historical bias, caused by change in practice, development of the learning curve and enhancement of peri-operative care. Clearly a randomization was not possible but neither was a selection: patients in both groups, study or control, all underwent LTG with the same technique, following the same oncology protocol. Perioperative care was different amongst the two groups but applied to all patients within its own group. Reduction in complications observed in group A could be attributable to both IN and/or ERAS. Nevertheless, the reduction of LOS could be attributed to both a reduction in complications’ rate, or to a change of perioperative management with the introduction of ERAS.
5 Conclusions
The implementation of ERAS and IN seems to reduce LOS and overall complications after LTG. Early feeding is well accepted even in the elderly.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Amelio I, Bertolo R, Bove P, Buonomo O, Candi E, et al. Liquid biopsies and cancer omics. Cell Death Discov. 2020;6(1):131. https://doi.org/10.1038/s41420-020-00373-0.
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424.
Mortensen K, Nilsson M, Slim K, Schäfer M, Mariette C, Braga M, Carli F, Demartines N, Griffin SM, Lassen K, Enhanced Recovery After Surgery (ERAS®) Group. Consensus guidelines for enhanced recovery after gastrectomy: enhanced recovery after surgery (ERAS®) society recommendations. Br J Surg. 2014;101(10):1209–29. https://doi.org/10.1002/bjs.9582.
Ljungqvist O, Scott M, Fearon KC. Enhanced recovery after surgery. JAMA Surg. 2017. https://doi.org/10.1001/jamasurg.2016.4952.
Fina D, Franzè E, Rovedatti L, Corazza GR, Biancone L, Sileri PP, Sica G, MacDonald TT, Pallone F, Di Sabatino A, Monteleone G. Interleukin-25 production is differently regulated by TNF-alpha and TGF-beta 1 in the human gut. Mucosal Immunol. 2014;4(2):239–44. https://doi.org/10.1038/mi.2010.68.
Zhang B, Najarali Z, Ruo L, Alhusaini A, Solis N, Valencia M, Sanchez MIP, Serrano PE. Effect of perioperative nutritional supplementation on postoperative complications—systematic review and meta-analysis. J Gastrointest Surg. 2019;23:1682–93. https://doi.org/10.1007/s11605-019-04173-5.
Probst P, Ohmann S, Klaiber U, Hüttner FJ, Billeter AT, Ulrich A, Büchler MW, Diener MK. Meta-analysis of immunonutrition in major abdominal surgery. Br J Surg. 2017;104:1594–608. https://doi.org/10.1002/bjs.10659.
Zheng HL, Lu J, Li P, et al. Effects of preoperative malnutrition on short-and long-term outcomes of patients with gastric cancer: can we do better? Ann Surg Oncol. 2017;24:3376–85. https://doi.org/10.1245/s10434-017-5998-9.
Cheng Y, Zhang J, Zhang L, et al. Enteral immunonutrition versus enteral nutrition for gastric cancer patients undergoing a total gastrectomy: a systematic review and meta-analysis. BMC Gastroenterol. 2018;18(1):11. https://doi.org/10.1186/s12876-018-0741-y.
Amin MB, Edge S, Greene F, Byrd DR, Brookland RK, Washington MK, Gershenwald JE, Compton CC, Hess KR, et al editors. AJCC cancer staging manual (8th edition). American Joint Commission on Cancer. Cham: Springer International Publishing; 2017.
Kaiser MJ, Bauer JM, Ramsch C, Uter W, Guigoz Y, Cederholm T, Thomas DR, Anthony P, Charlton KE, Maggio M, et al. Validation of the mini nutritional assessment short-form (MNA®-SF): a practical tool for identification of nutritional status. J Nutr Health Aging. 2009;13:782–8.
Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care–associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36(5):309–32.
Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004. https://doi.org/10.1097/01.sla.0000133083.54934.ae.
Von Elm E, Altman DG, Egger M, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Bull World Health Organ. 2007;85(11):867–72. https://doi.org/10.2471/blt.07.045120.
Cardi M, Sammartino P, Mingarelli V, Sibio S, Accarpio F, Biacchi D, Musio D, Sollazzo B, Di Giorgio A. Cytoreduction and HIPEC in the treatment of unconventional secondary peritoneal carcinomatosis. World J Surg Oncol. 2015;13:305. https://doi.org/10.1186/s12957-015-0703-6.
Nygren J, Thacker J, Carli F, Fearon KC, Norderval S, Lobo DN, et al. Guidelines for perioperative care in elective rectal/pelvic surgery: enhanced Recovery After Surgery (ERAS®) Society recommendations. Clin Nutr. 2012;31(6):801–16. https://doi.org/10.1016/j.clnu.2012.08.012.
Gustafsson UO, Scott MJ, Schwenk W, Demartines N, Roulin D, Francis N, et al. Guidelines for perioperative care in elective colonic surgery: enhanced recovery after surgery (ERAS®) society recommendations. World J Surg. 2013;37:259–84. https://doi.org/10.1007/s00268-012-1772-0.
Lassen K, Coolsen MM, Slim K, Carli F, de Aguilar-Nascimento JE, Shafer M, et al. Guidelines for perioperative care for pancreaticoduodenenctomy: enhanced Recovery After Surgery (ERAS®) Society recommendations. World J Surg. 2013;37:240–58. https://doi.org/10.1007/s00268-012-1771-1.
Gentileschi P, Camperchioli I, Benavoli D, Di Lorenzo N, Sica G, Gaspari AL. Laparoscopic single-port sleeve gastrectomy for morbid obesity: preliminary series. Surg Obes Relat Dis. 2010;6(6):665–9. https://doi.org/10.1016/j.soard.2010.01.011.
Ding J, Sun B, Song P, et al. The application of enhanced recovery after surgery (ERAS)/fast-track surgery in gastrectomy for gastric cancer a systematic review and meta-analysis. Oncotarget. 2017;8(43):75699–711. https://doi.org/10.18632/oncotarget.
Li Z, Wang Q, Li B, et al. Influence of enhanced recovery after surgery programs on laparoscopy-assisted gastrectomy for gastric cancer a systematic review and meta-analysis of randomized control trials. World J Surg Oncol. 2017;15:207. https://doi.org/10.1186/s12957-017-1271-8.
Li MZ, Wu WH, Li L, et al. Is ERAS effective and safe in laparoscopic gastrectomy for gastric carcinoma ? A meta-analysis. World J Surg Oncol. 2018;16:17. https://doi.org/10.1186/s12957-018-1309-6.
Wang LH, Zhu RF, Gao C, et al. Application of enhanced recovery after gastric cancer surgery: an updated meta-analysis. World J Gastroenterol. 2018;24(14):1562–11578. https://doi.org/10.3748/wjg.v24.i14.1562.
Lee Y, Yu J, Doumouras AG, Li J, Hong D. Enhanced recovery after surgery (ERAS) versus standard recovery for elective gastric cancer surgery: a meta-analysis of randomized controlled trials. Surg Oncol. 2020;32:75–87.
Wee IJY, Li-Xun Syn N, Shabbir A, Kim G, So JBY. Enhanced recovery versus conventional care in gastric cancer surgery: a meta-analysis of randomized and non-randomized controlled trials. Gastric Cancer. 2019;22:423–34.
Pisarska M, Pędziwiatr M, Major P, Kisielewski M, Migaczewski M, Rubinkiewicz M, Budzyński P, Przęczek K, Zub-Pokrowiecka A, Budzyński A. Laparoscopic gastrectomy with enhanced recovery after surgery protocol: single-center experience. Med Sci Monit. 2017;23(23):1421–7. https://doi.org/10.12659/msm.898848.
Song GM, Tian X, Liag H, et al. Role of enteral immunonutrition in patients undergoing surgery for gastric cancer: a systematic review and meta-analysis of randomized controlled trials. Medicine (Baltimore). 2015;94(31): e1311. https://doi.org/10.1097/MD.0000000000001311.
Makuuchi R, Sugisawa N, Kaji S, et al. Enhanced Recovery after surgery for gastric cancer and an assessment of preoperative carbohydrate loading. Eur J Surg Oncol. 2017;43:210–7.
Rossi P, Sileri P, Gentileschi P, et al. Percutaneous liver biopsy using an ultrasound-guided subcostal route. Dig Dis Sci. 2001;46(1):128–32. https://doi.org/10.1023/a:1005571904713.
Van Stijn MF, Korkic-Halilovic I, Bakker MS, van der Ploeg T, van Leeuwen PA, Houdij PJ. Preoperative nutrition status and postoperative outcome in elderly general surgery patients: a systematic review. JPEN J Parenter Enteral Nutr. 2013;37:37–43.
Cerantola Y, Hubner M, Grass F, Demartines N, Schafer M. Immunonutrition in gastrointestinal surgery. Br J Surg. 2011;98:37–48.
EuroSurg Collaborative. Body mass index and complications following major gastrointestinal surgery: a prospective, international cohort study and meta-analysis. Colorectal Dis. 2018;20(8):O215–25. https://doi.org/10.1111/codi.14292.
Zhang Y, Gu Y, Guo T, Li Y, Cai H. Perioperative immunonutrition for gastrointestinal cancer: a systematic review of randomized controlled trials. Surg Oncol. 2012;21:e87–95.
Sica GS, Djapardy V, Westaby S, Maynard ND. Diagnosis and management of aortoesophageal fistula caused by a foreign body. Ann Thorac Surg. 2004;77(6):2217–8. https://doi.org/10.1016/j.athoracsur.2003.06.031 (ISSN: 0003497).
Cao S, Zheng T, Wang H, Niu Z, Chen D, Zhang J, Lv L, Zhou Y. Enhanced recovery after surgery in elderly gastric cancer patients undergoing laparoscopic total gastrectomy. J Surg Res. 2021;257:579–86. https://doi.org/10.1016/j.jss.2020.07.037.
Fina D, Franzè E, Rovedatti L, Corazza GR, Biancone L, Sileri PP, Sica G, MacDonald TT, Pallone F, Di Sabatino A, Monteleone G. Interleukin-25 production is differently regulated by TNF-α and TGF-β1 in the human gut. Mucosal Immunol. 2011;4(2):239–44. https://doi.org/10.1038/mi.2010.68.
Sileri P, Mele A, Stolfi VM, Grande M, Sica G, Gentileschi P, Di Carlo S, Gaspari AL. Medical and surgical treatment of chronic anal fissure: a prospective study. J Gastrointest Surg. 2007;11(11):1541–8. https://doi.org/10.1007/s11605-007-0255-3.
Hegazi RA, Hustead DS, Evans DC. Preoperative standard oral nutrition supplements vs immunonutrition: results of a systematic review and meta-analysis. J Am Coll Surg. 2014;219(5):1078–87.
Zhang B, Najarali Z, Ruo L, Alhusaini A, Solis N, Valencia M, Pinto Sanchez MI, Serrano PE. Effect of perioperative nutritional supplementation on postoperative complications-systematic review and meta-analysis. J Gastrointest Surg. 2019;23:1682–93. https://doi.org/10.1007/s11605-019-04173-5.
Ida S, Hiki N, Cho H, Sakamaki K, Ito S, Fujitani K, et al. Randomized clinical trial comparing standard diet with perioperative oral immunonutrition in total gastrectomy for gastric cancer. BJS. 2017;104:377–83.
Song GM, Liu XL, Bian W, et al. Systematic review with network meta-analysis: comparative efficacy of different enteral immunonutrition formulas in patients underwent gastrectomy. Oncotarget. 2017;8(14):23376–88. https://doi.org/10.18632/oncotarget.15580.
Nepravishta R, Sabelli R, Iorio E, Micheli L, Paci M, Melino S. Oxidative species and S-glutathionyl conjugates in the apoptosis induction by allyl thiosulfate. FEBS J. 2012;279(1):154–67. https://doi.org/10.1111/j.1742-4658.2011.08407.x.
Yamagata Y, Yoshikawa T, Yura M, Otsuki S, Morita S, Katai H, Nishida T. “Current status of enhanced recovery after surgery” program in gastric cancer surgery. Ann Gastroenterol Surg. 2019;3(3):231–8. https://doi.org/10.1002/ags3.12232.
Desiderio J, Trastulli S, D’Andrea V, Parisi A. Enhanced recovery after surgery for gastric cancer (ERAS-GC): optmizing patient outcome. Transl Gastroenterol Hepatol. 2020;5(5):11. https://doi.org/10.21037/tgh.2019.10.04.
Wang Q, Guo BY, Zhao QC, Yan ZD, Shang LF, Yu J, Ji G. Safety of early oral feeding after total laparoscopic radical gastrectomy for gastric cancer (SOFTLY): study protocol for a randomized controlled trial. BMC. 2019;20:384. https://doi.org/10.1186/s13063-019-3493-2.
Tesauro M, Guida AM, Siragusa L, Sensi B, Bellato V, Di Daniele N, Divizia A, Franceschilli M, Sica GS. Preoperative Immunonutrition vs standard dietary advice in normo-nourished patients undergoing fast-track laparoscopic colorectal surgery. J Clin Med. 2021;10(3):413. https://doi.org/10.3390/jcm10030413.
Sica GS, Iaculli E, Biancone L, Di Carlo S, Scaramuzzo R, Fiorani C, Gentileschi P, Benavoli D, A.L. Gaspari W,. Comparative study of laparoscopic versus open gastrectomy in gastric cancer management. J Gastroenterol. 2011;17(41):4602–6. https://doi.org/10.3748/wjg.v17.i41.4602.
Funding
This research didn’t received grants from any founding agency in public, commercial or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
MF and SDC wrote the manuscript and made substantial direct equal intellectual contribution. MF, LS, VU, SD, BP, SS, and SDC. contributed to the conception, design, analysis and/or interpretation of data and they made substantial intellectual contribution. All the authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was conducted according to the guidelines of the Declaration of Helsinki. Ethical approval was not requested as for local policy on retrospective study conducted exclusively on datasets where involved subjects are not identifiable.
Informed consent was obtained from all subjects involved in the study. However, no patient can be identified in the present study.
Competing interests
This research didn’t received grants from any founding agency in public, commercial or not-for-profit sectors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Franceschilli, M., Siragusa, L., Usai, V. et al. Immunonutrition reduces complications rate and length of stay after laparoscopic total gastrectomy: a single unit retrospective study. Discov Onc 13, 62 (2022). https://doi.org/10.1007/s12672-022-00490-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12672-022-00490-5