Abstract
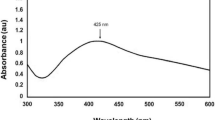
We report herein a one-step, biomimetic synthesis of silver nanoparticles (AgNPs) from Musa balbisiana leaf extract having a cytotoxic effect on SiHa and HL-60 cancer cell lines without affecting non-cancerous, healthy peripheral blood mononuclear cells (PBMCs). The EDX analysis of AgNP dispersion, with a peak of 3.2 keV, confirmed the presence of elemental silver with a weight percentage of 54.57%. As is revealed from both SEM and TEM, monodispersed AgNPs were spherical in morphology with a diameter ranging from 11.28 to 87.53 nm with an average size of 12 nm. The diffraction pattern revealed polycrystalline AgNPs with FCC crystal structure. We report increased cytotoxicity accompanied with characteristic morphological change in HL-60 and SiHa, significant reduction in proliferation, increased reactive oxygen species (ROS) generation, and decreased mitochondrial membrane potential (MMP) following AgNP treatment at various doses. No such detrimental cellular impact of AgNPs was observed on healthy PBMCs. Fluorescence microscopy tracked coumarin-loaded AgNP accumulation in lysosomes as well as the nucleus, suggesting that both lysosome and nucleus served as cellular targets of AgNPs. AgNPs were internalized through passive and active pathways and that energy-dependent, clathrin-mediated endocytosis was involved in the trafficking of AgNPs. Our in silico docking studies confirm the binding of AgNPs to adaptor protein 2 on the β2 subunit facilitating clathrin-mediated endocytosis.
Graphical abstract




















Similar content being viewed by others
References
Malekzad, H., Zangabad, P. S., Mirshekari, H., Karimi, M., & Hamblin, M. R. (2017). Noble metal nanoparticles in biosensors: Recent studies and applications. Nanotechnology Reviews, 6, 301–329. https://doi.org/10.1515/ntrev-2016-0014
Iravani, S., Korbekandi, H., Mirmohammadi, S. V., & Zolfaghari, B. (2014). Synthesis of silver nanoparticles. Chem phys and biological methods Res Pharm Sci, 9, 385–406.
Burdusel, A.C., Gherasim, O., Grumezescu, AM., Mogoanta, L., Ficai, A., & Andronescu, E. (2018). Biomedical Applications of Silver Nanoparticles: An Up-to-Date Overview. Nanomaterials (Basel), 8, 681. https://doi.org/10.3390/nano8090681
Yang, N. J., & Hinner, M. J. (2015). Getting across the cell membrane An overview for small molecules peptides and proteins. Methods Molecular Biology, 1266, 29–53. https://doi.org/10.1007/978-1-4939-2272-7_3
Salatin, S., & Yari Khosroushahi, A. (2017). Overviews on the cellular uptake mechanism of polysaccharide colloidal nanoparticles. Journal of Cellular and Molecular Medicine, 21, 1668–1686. https://doi.org/10.1111/jcmm.13110
Sahay, G., Alakhova, D. Y., & Kabanov, A. V. (2010). Endocytosis of nanomedicines J Control Release, 145, 182–195. https://doi.org/10.1016/j.jconrel.2010.01.036
Foroozandeh, P., & Aziz, A. A. (2018). Insight into cellular uptake and intracellular trafficking of nanoparticles. Nanoscale Research Letters, 1, 339. https://doi.org/10.1186/s11671-018-2728-6
Kumari, S., Mg, S., & Mayor, S. (2010). Endocytosis unplugged: Multiple ways to enter the cell. Cell Research, 20, 256–275. https://doi.org/10.1038/cr.2010.19
Kiss, A. L., & Botos, E. (2009). Endocytosis via caveolae: Alternative pathway with distinct cellular compartments to avoid lysosomal degradation? Journal of Cellular and Molecular Medicine, 13, 1228–1237. https://doi.org/10.1111/j.1582-4934.2009.00754.x
Popova, N. V., Deyev, I. E., & Petrenko, A. G. (2013). Clathrin-mediated endocytosis and adaptor proteins Acta Naturae, 5, 62–73.
Ahmed, S. (2016). Saifullah, Ahmad M, Swami BL, Ikram S. Green synthesis of silver nanoparticles using Azadirachta indica aqueous leaf extract J Radiat Res Appl Sci, 9, 1–7.
Song, Z., Chang, Y., Xie, H., Yu, X.-F., Chu, P. K., & Chen, T. (2017). Decorated ultrathin bismuth selenide nanosheets as targeted theranostic agents for in vivo imaging guided cancer radiation therapy NPG Asia Mater, 9, e439–e439. https://doi.org/10.1038/am.2017.167
Zhu, B., Li, Y., Lin, Z., Zhao, M., Xu, T., Wang, C., & Deng, N. (2016). Silver Nanoparticles induce HePG-2 cells apoptosis through ROS-mediated signaling pathways. Nanoscale Res Lett, 11, 198. https://doi.org/10.1186/s11671-016-1419-4
Elumalai, D., Hemavathi, M., Deepaa, C. V., & Kaleena, P. K. (2017). Evaluation of phytosynthesised silver nanoparticles from leaf extracts of Leucas aspera and Hyptis suaveolens and their larvicidal activity against malaria dengue and filariasis vectors. Parasite Epidemiol Control, 2, 15–26. https://doi.org/10.1016/j.parepi.2017.09.001
Li, J., Chen, J., Wang, H., Chen, N., Wang, Z., Guo, L., & Deepak, F. L. (2018). In situ atomic-scale study of particle-mediated nucleation and growth in amorphous bismuth to nanocrystal phase transformation. Advances Science (Weinh), 5, 1700992. https://doi.org/10.1002/advs.201700992
Giannini, C., Ladisa, M., Altamura, D., Siliqi, D., Sibillano, T., & De Caro, L. (2016). X-ray diffraction: A powerful technique for the multiple-length-scale structural analysis of nanomaterials. Crystals, 6, 87.
Shanmugam, C., Sivasubramanian, G., Parthasarathi, B., Baskaran, K., Balachander, R., & Parameswaran, V. R. (2015). Antimicrobial, free radical scavenging activities and catalytic oxidation of benzyl alcohol by nano-silver synthesized from the leaf extract of Aristolochia indica L. A promenade towards sustainability Applied Nanoscience, 6, 711–723. https://doi.org/10.1007/s13204-015-0477-8
Braydich-Stolle, L., Hussain, S., Schlager, J. J., & Hofmann, M. C. (2005). In vitro cytotoxicity of nanoparticles in mammalian germline stem cells. Toxicological Sciences, 88, 412–419. https://doi.org/10.1093/toxsci/kfi256
Cory, A. H., Owen, T. C., Barltrop, J. A., & Cory, J. G. (1991). Use of an aqueous soluble tetrazolium/formazan assay for cell growth assays in culture. Cancer Communications, 3, 207–212. https://doi.org/10.3727/095535491820873191
Jain, S., & Mehata, M. S. (2017). Medicinal plant leaf extract and pure flavonoid mediated green synthesis of silver nanoparticles and their enhanced antibacterial property. Science Reports, 7, 15867. https://doi.org/10.1038/s41598-017-15724-8
Noginov, M. A., et al. (2006). The effect of gain and absorption on surface plasmons in metal nanoparticles. Applied Physics B, 86, 455–460. https://doi.org/10.1007/s00340-006-2401-0
Christopher, J. G., Saswati, B., & Ezilrani, P. (2015). Optimization of parameters for biosynthesis of silver nanoparticles using leaf extract of Aegle marmelos. Brazilian Archives of Biology and Technology, 58, 702–710. https://doi.org/10.1590/s1516-89132015050106
Zhang, J., Tang, H., Liu, Z., & Chen, B. (2017). Effects of major parameters of nanoparticles on their physical and chemical properties and recent application of nanodrug delivery system in targeted chemotherapy. International Journal of Nanomedicine, 12, 8483–8493. https://doi.org/10.2147/ijn.S148359
Tang, L., et al. (2014). Investigating the optimal size of anticancer nanomedicine. Proceedings of the National Academy of Sciences, 111, 15344–15349. https://doi.org/10.1073/pnas.1411499111
Sarkar, S., & Kotteeswaran, V. (2018). Green synthesis of silver nanoparticles from aqueous leaf extract of Pomegranate (Punica granatum) and their anticancer activity on human cervical cancer cells. Advances in Natural Sciences: Nanoscience and Nanotechnology, 9, 025014. https://doi.org/10.1088/2043-6254/aac590
Jyoti, K., Baunthiyal, M., & Singh, A. (2019). Characterization of silver nanoparticles synthesized using Urtica dioica Linn leaves and their synergistic effects with antibiotics. Journal of Radiation Research and Applied Sciences, 9, 217–227. https://doi.org/10.1016/j.jrras.2015.10.002
Govindarajan, M., Vijayan, P., Kadaikunnan, S., Alharbi, N. S., & Benelli, G. (2016). One-pot biogenic fabrication of silver nanocrystals using Quisqualis indica Effectiveness on malaria and Zika virus mosquito vectors, and impact on non-target aquatic organisms. Journal of Photochemistry and Photobiology B Biology, 162, 646–655. https://doi.org/10.1016/j.jphotobiol.2016.07.036
Annamalai, A., Christina, V. L. P., Christina, V., & Lakshmi, P. T. V. (2012). Green synthesis and characterisation of Ag NPs using aqueous extract of Phyllanthus maderaspatensis L. Journal of Experimental Nanoscience, 9, 113–119. https://doi.org/10.1080/17458080.2011.631041
Hedaginal, B. R., & Taranath, T. C. (2016). Phytosynthesis of silver nanoparticles by Thunbergia fragrans Roxb. and their characterization Int. Journal Pharm Science Review Research., 39, 54–58.
Shang, L., Nienhaus, K., & Nienhaus, G. (2014). Engineered nanoparticles interacting with cells: size matters. Journal of Nanobiotechnology, 12, 5. https://doi.org/10.1186/1477-3155-12-5
AbdelRahim, K., Mahmoud, S. Y., Ali, A. M., Almaary, K. S., Mustafa, A.E.-Z.M.A., & Husseiny, S. M. (2017). Extracellular biosynthesis of silver nanoparticles using Rhizopus stolonifer Saudi. Journal of Biological Sciences, 24, 208–216. https://doi.org/10.1016/j.sjbs.2016.02.025
Peng, X., Rahman, M. A., Mao, H., & Shin, D. M. (2016). Nanomaterials facilitate tumor targeting and drug delivery. Austin J Cancer Clin Res, 3, 1072.
Hoshyar, N., Gray, S., Han, H., & Bao, G. (2016). The effect of nanoparticle size on in vivo pharmacokinetics and cellular interaction. Nanomedicine, 11, 673–692. https://doi.org/10.2217/nnm.16.5
Li, S., Shen, Y., Xie, A., Yu, X., Qiu, L., Zhang, L., & Zhang, Q. (2007). Green synthesis of silver nanoparticles using Capsicum annuum L. extract. Green Chemistry, 9, 852–858. https://doi.org/10.1039/b615357g
Cummings, B. S., & Schnellmann, R. G. (2004). Measurement of Cell Death in Mammalian Cells. Current Protocols in Pharmacology, 25, 12. https://doi.org/10.1002/0471141755.ph1208s25
Buttacavoli, M., Albanese, N. N., Di Cara, G., Alduina, R., Faleri, C., Gallo, M., Pizzolanti, G., Gallo, G., Feo, S., Baldi, F., & Cancemi, P. (2018) Anticancer activity of biogenerated silver nanoparticles: an integrated proteomic investigation. Oncotarget, 9, 9685–9705.
Carlson, C., Hussain, S. M., Schrand, A. M., Braydich-Stolle, K. L., Hess, K. L., Jones, R. L., & Schlager, J. J. (2008). Unique cellular interaction of silver nanoparticles Size-dependent generation of reactive oxygen species. The Journal of Physical Chemistry B, 112, 13608–13619. https://doi.org/10.1021/jp712087m
Gurunathan, S., Qasim, M., Park, C., Yoo, H., Kim, J-H., & Hong, K. (2018). Cytotoxic potential and molecular pathway analysis of silver nanoparticles in human colon cancer cells HCT116. International Journal of Molecular Sciences, 19, 2269. https://doi.org/10.3390/ijms19082269
Zamzami, N., Marchetti, P., Castedo, M., Zanin, C., Vayssière, J. L., Petit, P. X., & Kroemer, G. (1995). Reduction in mitochondrial potential constitutes an early irreversible step of programmed lymphocyte death in vivo. The Journal of Experimental Medicine, 181, 1661–1672. https://doi.org/10.1084/jem.181.5.1661
Giorgi, C., Baldassari, F., Bononi, A., Bonora, M., De Marchi, E., Marchi, S., Missiroli, S., Patergnani, S., Rimessi, A., Suski, J. M., Wieckowski, M. R., & Pinton, P. (2012). Mitochondrial Ca2+ and apoptosis. Cell Calcium, 52, 36–43. https://doi.org/10.1016/j.ceca.2012.02.008
Lizarbe, M., Barrasa, J., Olmo, N., Gavilanes, F., & Turnay, J. (2013). Annexin-phospholipid interactions. Functional Implications International Journal of Molecular Sciences, 14, 2652–2683. https://doi.org/10.3390/ijms14022652
Plackal Adimuriyil George, B., Kumar, N., Abrahamse, H., & Ray, S. S. (2018). Apoptotic efficacy of multifaceted biosynthesized silver nanoparticles on human adenocarcinoma cells. Scientific Reports, 8, 14368. https://doi.org/10.1038/s41598-018-32480-5
Miyayama, T., & Matsuoka, M. (2016). Involvement of lysosomal dysfunction in silver nanoparticle-induced cellular damage in A549 human lung alveolar epithelial cells. Journal of Occupational Medicine and Toxicology, 11, 1. https://doi.org/10.1186/s12995-016-0090-0
Boya, P., & Kroemer, G. (2008). Lysosomal membrane permeabilization in cell death Oncogene, 27, 6434–6451. https://doi.org/10.1038/onc.2008.310
Panzarini, E., Mariano, S., Carata, E., Mura, F., Rossi, M., & Dini, L. (2018). Intracellular transport of silver and gold nanoparticles and biological responses: An update. International Journal of Molecular Sciences, 19, 1305. https://doi.org/10.3390/ijms19051305
Lu, J., et al. (2008). Mesoporous Silica nanoparticles for cancer therapy: Energy-dependent cellular uptake and delivery of paclitaxel to cancer cells. NanoBiotechnology, 3, 89–95. https://doi.org/10.1007/s12030-008-9003-3
Goldberg, M., & Gomez-Orellana, I. (2003). Challenges for the oral delivery of macromolecules. Nature Reviews Drug Discovery, 2, 289–295. https://doi.org/10.1038/nrd1067
Harvey, J., Hardy, S. C., & Ashford, M. L. J. (1999). Dual actions of the metabolic inhibitor, sodium azide on KATPchannel currents in the rat CRI-G1 insulinoma cell line. British Journal of Pharmacology, 126, 51–60. https://doi.org/10.1038/sj.bjp.0702267
Kim, J. S., Yoon, T. J., Yu, K. N., Noh, M. S., Woo, M., Kim, B. G., Lee, K. H., Sohn, B. H., Park, S. B., Lee, J. K., & Cho, M. H. (2006). Cellular uptake of magnetic nanoparticle is mediated through energy-dependent endocytosis in A549 cells. Journal of Veterinary Science, 7, 321–326. https://doi.org/10.4142/jvs.2006.7.4.321
Meng, X.-Y., Zhang, H.-X., Mezei, M., & Cui, M. (2011). Molecular docking: A powerful approach for structure-based drug discovery. Current Computer Aided-Drug Design, 7, 146–157. https://doi.org/10.2174/157340911795677602
Jackson, L. P., Kümmel, D., Reinisch, K. M., & Owen, D. J. (2012). Structures and mechanisms of vesicle coat components and multisubunit tethering complexes. Current Opinion in Cell Biology, 24, 475–483. https://doi.org/10.1016/j.ceb.2012.05.013
Schmid, S. L., Reed, D. K., Mohanakrishnan, A., Loerke, D., Spielman, S. J., & Kadlecova, Z. (2017). Regulation of clathrin-mediated endocytosis by hierarchical allosteric activation of AP2. Journal of Cell Biology, 216, 167–179. https://doi.org/10.1083/jcb.201608071
Nnyigide, O. S., Lee, S-G., & Hyun, K. (2019). In silico characterization of the binding modes of surfactants with bovine serum albumin. Scientific Reports, 9, 10643. https://doi.org/10.1038/s41598-019-47135-2
Funding
This research was supported by the DST-PURSE Programme (5050).
Department of Science and Technology,Ministry of Science and Technology,DST-PURSE Programme (5050),Geeta Rai
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Research Involving Humans and Animals Statement
PBMCs were isolated from whole blood obtained from three healthy donors after informed and written consent. Ethical consent for the use of human samples was obtained from the ethics committee of the Institute of Medical Sciences, Banaras Hindu University.
Informed Consent
Written and informed consent was obtained from healthy donors.
Ethical Statement
The authors state that the submitted work is original and not published elsewhere in any form or language. The presented data are original, honest, and without fabrication, falsification, or inappropriate data manipulation.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Priya, K., Das, D., Singh, S. et al. Green Synthesis of Silver Nanoparticles Using Musa balbisiana and Their Cytotoxic Effect on HL-60 and SiHa Cancer Cells Through Clathrin-Mediated Endocytosis. BioNanoSci. 12, 582–600 (2022). https://doi.org/10.1007/s12668-022-00955-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12668-022-00955-5