Abstract
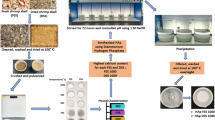
Precious materials obtained from biowaste have risen the attentions toward extracting and using these materials in various applications to address both economic and environmental demands. In the present study, we focused on the extraction of hydroxyapatite (HA) from fish bones through the thermal calcination method. The primary tests were carried out to characterize the fish bone-derived materials in terms of chemical composition, morphology, and viability. Therefore, series of characterizing tests including Fourier transform infrared spectroscopy (FT-IR), X-ray diffraction (XRD), and inductively coupled plasma (ICP) were carried out. Also, secondary electron microscopy equipped with energy-dispersive spectroscopy (EDS) was performed to determine morphology and elemental analysis of the obtained powder. Furthermore, the cell viability of fish bone-derived hydroxyapatite (FHA) along with its cell differentiation capability was evaluated by performing MTT and ALP assays and the results were compared with those of the commercial type of hydroxyapatite (CHA). The secondary phase of the current study relates to the capability of FHA and CHA on adsorbing nickel, as a model heavy metal, from aqueous solutions by performing bath adsorption experiments and considering initial concentration of nickel, adsorbent dosage, and contact time as the variables. In order to investigate kinetic model and adsorption mechanism, first-order, pseudo-second-order, and intraparticle diffusion kinetic models were used. Also, the equilibrium data were analyzed using Langmuir, Freundlich, and DKR adsorption isotherm models. The XRD and FT-IR results confirmed the successful extraction of HA from fish bones. Besides, ICP and EDS results revealed that the Ca/P ratio of FHA was higher than that of stoichiometric ratio (1.67). In addition, the MTT and ALP results indicated that FHA seemed to be a viable material for cell proliferation and differentiation. Besides, the adsorption outcomes indicated that FHA was sufficiently capable of adsorbing nickel. It was observed that the adsorption data were fitted well with pseudo-second-order and Langmuir isotherm models with maximum adsorption capacities of 50.25 and 48.78 mg g−1on FHA and CHA, respectively.






Similar content being viewed by others
References
F. Yearbook, Fishery and aquaculture Statistics, 2013.
Elvevoll, E. O., & James, D. (2001). Nutrition and Health, 15, 155–167.
Ferraro, V., Cruz, I. B., Jorge, R. F., Malcata, F. X., Pintado, M. E., & Castro, P. M. (2010). Food Research International, 43, 2221–2233.
Gumisiriza, R., Mshandete, A. M., Rubindamayugi, F., Kansiime, F., & Kivaisi, A. K. (2009). African Journal of Environmental Science and Technology, 3, 013–020.
Pati, F., Adhikari, B., & Dhara, S. (2010). Bioresource Technology, 101, 3737–3742.
H.-J. Chai, J.-H. Li, H.-N. Huang, T.-L. Li, Y.-L. Chan, C.-Y. Shiau, C.-J. Wu, Journal of BioMed Research, 2010 (2010).
Chen, S., Hirota, N., Okuda, M., Takeguchi, M., Kobayashi, H., Hanagata, N., & Ikoma, T. (2011). Acta Biomaterialia, 7, 644–652.
Boutinguiza, M., Lusquiños, F., Comesaña, R., Riveiro, A., Quintero, F., & Pou, J. (2007). Applied Surface Science, 254, 1264–1267.
Boutinguiza, M., Lusquiños, F., Riveiro, A., Comesaña, R., & Pou, J. (2009). Applied Surface Science, 255, 5382–5385.
Suchanek, W., & Yoshimura, M. (1998). Journal of Materials Research, 13, 94–117.
H. Aoki, Science and medical applications of hydroxyapatite, Ishiyaku Euroamerica, 1991.
Hench, L. L. (1991). Journal of the American Ceramic Society, 74, 1487–1510.
Best, S., Porter, A., Thian, E., & Huang, J. (2008). Journal of the European Ceramic Society, 28, 1319–1327.
Zhang, H.-b., Zhou, K.-c., Li, Z.-y., & Huang, S.-p. (2009). Journal of Physics and Chemistry of Solids, 70, 243–248.
Rhee, S.-H. (2002). Biomaterials, 23, 1147–1152.
Pang, Y., & Bao, X. (2003). Journal of the European Ceramic Society, 23, 1697–1704.
Xu, J., Khor, K. A., Dong, Z., Gu, Y., Kumar, R., & Cheang, P. (2004). Materials Science and Engineering: A, 374, 101–108.
Tseng, Y.-H., Kuo, C.-S., Li, Y.-Y., & Huang, C.-P. (2009). Materials Science and Engineering: C, 29, 819–822.
Herliansyah, M., Hamdi, M., Ide-Ektessabi, A., Wildan, M., & Toque, J. (2009). Materials Science and Engineering: C, 29, 1674–1680.
Ooi, C., Hamdi, M., & Ramesh, S. (2007). Ceramics International, 33, 1171–1177.
Murugan, R., Ramakrishna, S., & Panduranga Rao, K. (2006). Materials Letters, 60, 2844–2847.
Huang, Y.-C., Hsiao, P.-C., & Chai, H.-J. (2011). Ceramics International, 37, 1825–1831.
Boutinguiza, M., Pou, J., Comesaña, R., Lusquiños, F., de Carlos, A., & León, B. (2012). Materials Science and Engineering: C, 32, 478–486.
Haberko, K., Bućko, M. M., Brzezińska-Miecznik, J., Haberko, M., Mozgawa, W., Panz, T., Pyda, A., & Zarębski, J. (2006). Journal of the European Ceramic Society, 26, 537–542.
Xiaoying, L., Yongbin, F., Duchun, G., Wei, C., & Eng, K. (2007). Mater, 342, 343.
Pallela, R., Venkatesan, J., & Kim, S. K. (2011). Ceramics International, 37, 3489–3497.
Piccirillo, C., Silva, M., Pullar, R., Braga da Cruz, I., Jorge, R., Pintado, M., & Castro, P. M. (2013). Materials Science and Engineering: C, 33, 103–110.
Kongsri, S., Janpradit, K., Buapa, K., Techawongstien, S., & Chanthai, S. (2013). Chemical Engineering Journal, 215, 522–532.
Mobasherpour, I., Salahi, E., & Pazouki, M. (2012). Arabian Journal of Chemistry, 5, 439–446.
Stötzel, C., Müller, F., Reinert, F., Niederdraenk, F., Barralet, J., & Gbureck, U. (2009). Colloids and Surfaces B: Biointerfaces, 74, 91–95.
Feng, Y., Gong, J.-L., Zeng, G.-M., Niu, Q.-Y., Zhang, H.-Y., Niu, C.-G., Deng, J.-H., & Yan, M. (2010). Chemical Engineering Journal, 162, 487–494.
Sugiyama, S., Matsumoto, H., Hayashi, H., & Moffat, J. B. (2000). Colloids and Surfaces A: Physicochemical and Engineering Aspects, 169, 17–26.
O’Connell, D. W., Birkinshaw, C., & O’Dwyer, T. F. (2008). Bioresource Technology, 99, 6709–6724.
Öztürk, A., Artan, T., & Ayar, A. (2004). Colloids and Surfaces B: Biointerfaces, 34, 105–111.
Mobasherpour, I., Salahi, E., & Pazouki, M. (2011). Journal of Saudi Chemical Society, 15, 105–112.
Liao, C.-J., Lin, F.-H., Chen, K.-S., & Sun, J.-S. (1999). Biomaterials, (19), 1807–1813.
C. Suryanarayana, M.G. Norton, X-ray diffraction: a practical approach, Springer, 1998.
Smiciklas, I., Onjia, A., Raicevic, S., Janackovic, D., Mitric, M., & Hazard, J. (2008). Mater, 152, 876.
LeGeros RZ, Legeros JP, Phosphate minerals, 45(1984) 351–385.
Stötzel, C., Müller, F. A., Reinert, F., Niederdraenk, F., Barralet, J. E., & Gbureck, U. (2009). Colloids and Surfaces B: Biointerfaces, 74, 91–95.
Gupta, N., Kushwaha, A. K., & Chattopadhyaya, M. (2012). Journal of the Taiwan Institute of Chemical Engineers, 43, 604–613.
Ho, Y., & McKay, G. (1998). Process Safety and Environmental Protection, 76, 332–340.
Lin, K., Pan, J., Chen, Y., Cheng, R., & Xu, X. (2009). Journal of Hazardous Materials, 161, 231–240.
Özcan, A. S., & Özcan, A. (2004). Journal of Colloid and Interface Science, 276, 39–46.
Giles, C. H., MacEwan, T. H., & Nakhwa, S. N. (1960). Smith D. Journal of the Chemical Society, 3973–3993.
Foroughi-dahr, M., Abolghasemi, H., Esmaieli, M., Nazari, G., & Rasem, B. (2015). Process Safety and Environmental Protection, 95(226–236.
Ketcha Mbadcam, J., & Anagho, S. (2011). Journal of Environmental Chemistry and Ecotoxicology, 3, 290–297.
Augustine, A., Orike, B., & Edidiong, A. (2007). EJEAFChe, 6, 2221–2234.
Ketcha Mbadcam, J., Dongmo, S., & Dinka’a Ndaghu, D. (2012). International Journal of Current Research, 4, 162–167.
Wang, C.-C., Juang, L.-C., Lee, C.-K., Hsu, T.-C., Lee, J.-F., & Chao, H.-P. (2004). Journal of Colloid and Interface Science, 280, 27–35.
Liu, Z. R., & Zhou, S. Q. (2010). Process Safety and Environmental Protection, 88, 62–66.
Fouladgar, M., Beheshti, M., & Sabzyan, H. (2015). Equilibrium and kinetic modeling. Journal of Molecular Liquids, 30, 1060–1073.
Mangaleshwaran, L., Thirulogachandar, A., Rajasekar, V., Muthukumaran, C., & Rasappan, K. (2015). Journal of the Taiwan Institute of Chemical Engineers, 55(112–118.
Ewecharoen, A., Thiravetyan, P., & Nakbanpote, W. (2008). Chemical Engineering Journal, 137, 181–188.
Kyzas, G. Z., & Kostoglou, M. (2015). Separation and Purification Technology, 149(92–102.
Wu, Y., Luo, H., Wang, H., Zhang, L., Liu, P., & Feng, L. (2014). Journal of Colloid and Interface Science, 436, 90–98.
Oter, O., & Akcay, H. (2007). Water Environment Research, 1, 329–335.
Katsou, E., Malamis, S., Haralambous, K. J., & Loizidou, M. (2010). Journal of Membrane Science, 360, 234–249.
Jha, V. K., Matsuda, M., & Miyake, M. (2008). Journal of Hazardous Materials, 160, 148–153.
Ghaee, A., Shariaty-Niassar, M., Barzin, J., & Zarghan, A. (2012). Applied Surface Science, 258, 7732–7743.
Aksu, Z., Açıkel, Ü., Kabasakal, E., & Tezer, S. (2002). Water Research, 36, 3063–3073.
Author information
Authors and Affiliations
Corresponding author
Additional information
Highlights
• Fish bone-derived hydroxyapatite (FHA) powder was obtained via thermal calcination method.
• The extraction method is easy, economical, and favorable for environmental concerns.
• The FHA powder showed proper cell viability compared to commercial hydroxyapatite (CHA).
• Both FHA and CHA powders expressed almost similar performance in the adsorption of nickel.
• The experimental results were in accordance with pseudo-second-order equation.
• The nickel adsorption parameters were well fitted with Langmuir equations.
Rights and permissions
About this article
Cite this article
Dabiri, S.M.H., Rezaie, A.A., Moghimi, M. et al. Extraction of Hydroxyapatite from Fish Bones and Its Application in Nickel Adsorption. BioNanoSci. 8, 823–834 (2018). https://doi.org/10.1007/s12668-018-0547-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12668-018-0547-y