Abstract
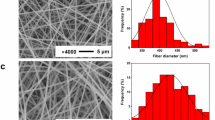
Electrospinning of chitosan-gelatin, using organic solvents, have already been reported. This study was designed to fabricate, characterize, and optimize electrospun chitosan-gelatin nanofibrous membranes using acetic acid as a more safe alternative aqueous solvent. Series of 90/10, 80/20, 70/30, 60/40, and 50/50 ratios of chitosan and gelatin were prepared using aqueous acetic acid as solvent. Blend solutions were electrospun after adding polyethylene oxide (PEO) to facilitate the electrospinning process. The effects of solution properties as well as the operating parameters on the architecture of the electrospun chitosan-gelatin nanofibers were investigated through evaluation of the electrical conductivity, viscosity, morphology, Fourier transform infrared spectroscopy (FTIR), mechanical properties, and hydrophilicity. Uniform beadless nanofibrous hydrophilic mats were fabricated with average fiber diameters from 229.79 ± 41.45 to 308.66 ± 50.03 nm. Concentration was the main parameter among all solution properties in controlling nanofiber diameter. The fiber diameters decreased with increasing the voltage, decreasing the feed rate, and increasing the needle to collector distance up to a certain amount. The interactions between the components of the blend solutions were confirmed by FTIR spectra. The Young’s moduli of all chitosan-gelatin blend nanofibers were higher than the chitosan nanofibers and increased significantly after cross-linking (P < 0.05). Chitosan-gelatin blends with different ratios of each biopolymer can be electrospun using acetic acid as an aqueous solvent and addition of PEO to yield hydrophilic membranes with uniform and beadless nanofibers. The architectural similarity of the nanofibrous chitosan-gelatin mats to natural ECM makes them great candidates for different biomedical applications.










Similar content being viewed by others
References
Garg, K., & Bowlin, G. L. (2011). Electrospinning jets and nanofibrous structures. Biomicrofluidics, 5(1), 013403. https://doi.org/10.1063/1.3567097.
Bhardwaj, N., & Kundu, S. C. (2010). Electrospinning: A fascinating fiber fabrication technique. Biotechnology Advances, 28(3), 325–347. https://doi.org/10.1016/j.biotechadv.2010.01.004.
La Mantia, F. P., Morreale, M., Botta, L., Mistretta, M. C., Ceraulo, M., & Scaffaro, R. (2017). Degradation of polymer blends: A brief review. Polym Degrad Stab 145 (Supplement C), 79–92. https://doi.org/10.1016/j.polymdegradstab.2017.07.011.
Theocharis, A. D., Skandalis, S. S., Gialeli, C., & Karamanos, N. K. (2016). Extracellular matrix structure. Adv Drug Del Rev, 97, 4–27. https://doi.org/10.1016/j.addr.2015.11.001.
Su, K., & Wang, C. (2015). Recent advances in the use of gelatin in biomedical research. Biotechnology Letters, 37(11), 2139–2145. https://doi.org/10.1007/s10529-015-1907-0.
Frantz, C., Stewart, K. M., & Weaver, V. M. (2010). The extracellular matrix at a glance. Journal of Cell Science, 123(24), 4195–4200. https://doi.org/10.1242/jcs.023820.
Pandey, A. R., Singh, U. S., Momin, M., & Bhavsar, C. (2017). Chitosan: Application in tissue engineering and skin grafting. Journal of Polymer Research, 24(8), 125. https://doi.org/10.1007/s10965-017-1286-4.
Ahmed, S., & Ikram, S. (2016). Chitosan based scaffolds and their applications in wound healing. Achiev Life Sci, 10(1), 27–37. https://doi.org/10.1016/j.als.2016.04.001.
Mirzaei, E., Faridi-Majidi, R., Shokrgozar, M. A., & Asghari Paskiabi, F. (2014). Genipin cross-linked electrospun chitosan-based nanofibrous mat as tissue engineering scaffold. Nanomed J, 1(3), 137–146. https://doi.org/10.7508/nmj.2014.03.003.
Dai, T., Tanaka, M., Huang, Y.-Y., & Hamblin, M. R. (2011). Chitosan preparations for wounds and burns: Antimicrobial and wound-healing effects. Expert Review of Anti-Infective Therapy, 9(7), 857–879. https://doi.org/10.1586/eri.11.59.
Haider, S., Al-Masry, W. A., Bukhari, N., & Javid, M. (2010). Preparation of the chitosan containing nanofibers by electrospinning chitosan–gelatin complexes. Polymer Engineering & Science, 50(9), 1887–1893. https://doi.org/10.1002/pen.21721.
Wang, S., & Zhao, G. (2012). Quantitative characterization of the electrospun gelatin–chitosan nanofibers by coupling scanning electron microscopy and atomic force microscopy. Materials Letters, 79, 14–17. https://doi.org/10.1016/j.matlet.2012.03.044.
Dhandayuthapani, B., Krishnan, U. M., & Sethuraman, S. (2010). Fabrication and characterization of chitosan-gelatin blend nanofibers for skin tissue engineering. Journal of Biomedical Materials Research Part B: Applied Biomaterials, 94B(1), 264–272. https://doi.org/10.1002/jbm.b.31651.
Pezeshki-Modaress, M., Zandi, M., & Mirzadeh, H. (2015). Fabrication of gelatin/chitosan nanofibrous scaffold: Process optimization and empirical modeling. Polymer International, 64(4), 571–580. https://doi.org/10.1002/pi.4843.
Charernsriwilaiwat, N., Opanasopit, P., Rojanarata, T., Ngawhirunpat, T., & Supaphol, P. (2010). Preparation and characterization of chitosan-hydroxybenzotriazole/polyvinyl alcohol blend nanofibers by the electrospinning technique. Carbohydrate Polymers, 81(3), 675–680. https://doi.org/10.1016/j.carbpol.2010.03.031.
Jafari, J., Emami, S. H., Samadikuchaksaraei, A., Bahar, M. A., & Gorjipour, F. (2011). Electrospun chitosan-gelatin nanofiberous scaffold: Fabrication and in vitro evaluation. Bio-medical Materials and Engineering, 21(2), 99–112. https://doi.org/10.3233/bme-2011-0660.
Zheng, Y., & Wyman, I. (2016). Supramolecular nanostructures based on Cyclodextrin and poly(ethylene oxide): Syntheses, structural characterizations and applications for drug delivery. Polymers, 8(5), 198.
Ma, L., Deng, L., & Chen, J. (2014). Applications of poly(ethylene oxide) in controlled release tablet systems: A review. Drug Development and Industrial Pharmacy, 40(7), 845–851. https://doi.org/10.3109/03639045.2013.831438.
Frohbergh, M. E., Katsman, A., Botta, G. P., Lazarovici, P., Schauer, C. L., Wegst, U. G., & Lelkes, P. I. (2012). Electrospun hydroxyapatite-containing chitosan nanofibers crosslinked with genipin for bone tissue engineering. Biomaterials, 33(36), 9167–9178. https://doi.org/10.1016/j.biomaterials.2012.09.009.
Li, Q., Wang, X., Lou, X., Yuan, H., Tu, H., Li, B., & Zhang, Y. (2015). Genipin-crosslinked electrospun chitosan nanofibers: Determination of crosslinking conditions and evaluation of cytocompatibility. Carbohydrate Polymers, 130, 166–174. https://doi.org/10.1016/j.carbpol.2015.05.039.
Chen, Z., Mo, X., He, C., & Wang, H. (2008). Intermolecular interactions in electrospun collagen–chitosan complex nanofibers. Carbohydrate Polymers, 72(3), 410–418. https://doi.org/10.1016/j.carbpol.2007.09.018.
Acevedo, C. A., Díaz-Calderón, P., López, D., & Enrione, J. (2015). Assessment of gelatin–chitosan interactions in films by a chemometrics approach. CyTA Journal of Food, 13(2), 227–234. https://doi.org/10.1080/19476337.2014.944570.
Pakravan, M., Heuzey, M.-C., & Ajji, A. (2011). A fundamental study of chitosan/PEO electrospinning. Polymer, 52(21), 4813–4824. https://doi.org/10.1016/j.polymer.2011.08.034.
Rahman, M. A., Khan, M. A., & Tareq, S. M. (2010). Preparation and characterization of polyethylene oxide (PEO)/gelatin blend for biomedical application: Effect of gamma radiation. Journal of Applied Polymer Science, 117(4), 2075–2082. https://doi.org/10.1002/app.32034.
Rakkapao, N., Vao-soongnern, V., Masubuchi, Y., & Watanabe, H. (2011). Miscibility of chitosan/poly(ethylene oxide) blends and effect of doping alkali and alkali earth metal ions on chitosan/PEO interaction. Polymer, 52(12), 2618–2627. https://doi.org/10.1016/j.polymer.2011.03.044.
Pillay, V., Dott, C., Choonara, Y. E., Tyagi, C., Tomar, L., Kumar, P., du Toit, L. C., & Ndesendo, V. M. K. (2013). A review of the effect of processing variables on the fabrication of electrospun nanofibers for drug delivery applications. Journal of Nanomaterials, 2013(22). https://doi.org/10.1155/2013/789289.
Bizarria, M. T. M., d'Ávila, M. A., & Mei, L. H. I. (2014). Non-woven nanofiber chitosan/peo membranes obtained by electrospinning. Brazilian Journal of Chemical Engineering, 31, 57–68.
Miri, M. A., Movaffagh, J., Najafi, M. B. H., Najafi, M. N., Ghorani, B., & Koocheki, A. (2016). Optimization of elecrospinning process of zein using central composite design. Fiber Polym, 17(5), 769–777. https://doi.org/10.1007/s12221-016-6064-0.
Veleirinho, B., Rei, M. F., & Lopes-Da-Silva, J. A. (2008). Solvent and concentration effects on the properties of electrospun poly(ethylene terephthalate) nanofiber mats. Journal of Polymer Science Part B: Polymer Physics, 46(5), 460–471. https://doi.org/10.1002/polb.21380.
Okutan, N., Terzi, P., & Altay, F. (2014). Affecting parameters on electrospinning process and characterization of electrospun gelatin nanofibers. Food Hydrocolloids, 39, 19–26. https://doi.org/10.1016/j.foodhyd.2013.12.022.
Thompson, C. J., Chase, G. G., Yarin, A. L., & Reneker, D. H. (2007). Effects of parameters on nanofiber diameter determined from electrospinning model. Polymer, 48(23), 6913–6922. https://doi.org/10.1016/j.polymer.2007.09.017.
Haider A, Haider S,Kang I-K (2015) A comprehensive review summarizing the effect of electrospinning parameters and potential applications of nanofibers in biomedical and biotechnology. ARAB J CHEM. https://doi.org/10.1016/j.arabjc.2015.11.015
Han, W., Minhao, L., Xin, C., Junwei, Z., Xindu, C., & Ziming, Z. (2015). Study of deposition characteristics of multi-nozzle near-field electrospinning in electric field crossover interference conditions. AIP Advances, 5(4), 041302. https://doi.org/10.1063/1.4902173.
Su, P., Wang, C., Yang, X., Chen, X., Gao, C., Feng, X.-X., Chen, J.-Y., Ye, J., & Gou, Z. (2011). Electrospinning of chitosan nanofibers: The favorable effect of metal ions. Carbohydrate Polymers, 84(1), 239–246. https://doi.org/10.1016/j.carbpol.2010.11.031.
Cebi, N., Durak, M. Z., Toker, O. S., Sagdic, O., & Arici, M. (2016). An evaluation of Fourier transforms infrared spectroscopy method for the classification and discrimination of bovine, porcine and fish gelatins. Food Chemistry, 190(Supplement C), 1109–1115. https://doi.org/10.1016/j.foodchem.2015.06.065.
Jalaja, K., & James, N. R. (2015). Electrospun gelatin nanofibers: A facile cross-linking approach using oxidized sucrose. International Journal of Biological Macromolecules, 73, 270–278. https://doi.org/10.1016/j.ijbiomac.2014.11.018.
Reddy, N., Reddy, R., & Jiang, Q. (2015). Crosslinking biopolymers for biomedical applications. Trends in Biotechnology, 33(6), 362–369. https://doi.org/10.1016/j.tibtech.2015.03.008.
V, J. L., Wei, S., & S, C. L. (2008). Crosslinked, electrospun chitosan–poly(ethylene oxide) nanofiber mats. Journal of Applied Polymer Science, 109(2), 968–975. https://doi.org/10.1002/app.28107.
Schiffman, J. D., & Schauer, C. L. (2007). Cross-linking chitosan nanofibers. Biomacromolecules, 8(2), 594–601. https://doi.org/10.1021/bm060804s.
Jing, X., Mi, H. Y., Peng, J., Peng, X. F., & Turng, L. S. (2015). Electrospun aligned poly(propylene carbonate) microfibers with chitosan nanofibers as tissue engineering scaffolds. Carbohydrate Polymers, 117, 941–949. https://doi.org/10.1016/j.carbpol.2014.10.025.
Denis, P. D., Ian, S. M., Malika, A., & William, M. G. (2010). Effect of surface wettability and topography on the adhesion of osteosarcoma cells on plasma-modified polystyrene. Journal of Biomaterials Applications, 26(3), 327–347. https://doi.org/10.1177/0885328210372148.
Tallawi, M., Rosellini, E., Barbani, N., Cascone, M. G., Rai, R., Saint-Pierre, G., & Boccaccini, A. R. (2015). Strategies for the chemical and biological functionalization of scaffolds for cardiac tissue engineering: A review. J R Soc Interface, 12(108), 20150254. https://doi.org/10.1098/rsif.2015.0254.
Sousa, I., Mendes, A., & Bártolo, P. J. (2013). PCL scaffolds with collagen bioactivator for applications in tissue engineering. Procedia Engineer 59 (Supplement C), 279–284. https://doi.org/10.1016/j.proeng.2013.05.122.
Halake, K., Kim, H. J., Birajdar, M., Kim, B. S., Bae, H., Lee, C., Kim, Y. J., Kim, S., Ahn, S., An, S. Y., Jung, S. H., & Lee, J. (2016). Recently developed applications for natural hydrophilic polymers. J Ind Eng Chem 40 (Supplement C), 16–22. https://doi.org/10.1016/j.jiec.2016.06.011.
Acknowledgements
This study was part of a research project which was financially supported by the vice chancellor for research of Mashhad University of Medical Sciences (No. 931634).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Amiri, N., Rozbeh, Z., Afrough, T. et al. Optimization of Chitosan-Gelatin Nanofibers Production: Investigating the Effect of Solution Properties and Working Parameters on Fibers Diameter. BioNanoSci. 8, 778–789 (2018). https://doi.org/10.1007/s12668-018-0540-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12668-018-0540-5