Abstract
Objectives
The management of hepatitis C has progressed from interferon-based therapy to oral direct acting antiviral therapy. Deranged lipid levels (total cholesterol, triglyceride) after treatment with interferon-based therapy are well known. There is a paucity of data on changes in lipid profile, glycemic parameters and alteration in quality of life with the newer regimen. This study was designed to assess the changes in lipid profile, glycemic parameters, quality of life in chronic hepatitis C patients with genotype 3 after treatment with sofosbuvir and daclatasvir.
Methods
The study was a single-centre, prospective study, conducted at tertiary care hospital from January 2017 to December 2017. Fifty patients, who received sofosbuvir (400 mg) and daclatasvir (60 mg) orally once daily for a period of 12 weeks for chronic hepatitis C and genotype 3, were recruited.
Results
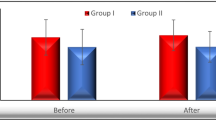
Total cholesterol levels (166.9 ± 23.8 to 192.4 ± 34.5 mg/dL, p-value < 0.0001) and low-density cholesterol (LDL) levels (100.9 ± 22.8 to 121.6 ± 37.2, p-value < 0.0001) were elevated after the treatment. A significant decrease in median levels of glycated hemoglobin (HbA1c) was observed (5.57% to 5.41%, p-value < 0.002). Quality of life markedly improved in all domains, i.e. physical, physiological, environmental, and social relationships according to an abbreviated form of World Health Organization quality of life assessment named WHOQOL-BREF questionnaire. Treatment was found to be effective with sustained virological response (SVR) achieved in 94% patients.
Conclusions
Our study reports a substantial increment in total cholesterol, and low-density lipoprotein with sofosbuvir and daclatasvir treatment though it achieved SVR in 94% of patients and improved their quality of life.
Similar content being viewed by others
References
Kim CW, Chang KM. Hepatitis C virus: virology and life cycle. Clin Mol Hepatol. 2013;19:17–25.
Smith DB, Bukh J, Kuiken C, et al. Expanded classification of hepatitis C virus into 7 genotypes and 67 subtypes: updated criteria and genotype assignment web resource. Hepatology. 2014;59:318–27.
Sundaram V. Dual daclatasvir and sofosbuvir for treatment of genotype 3 chronic hepatitis C virus infection. Expert Rev Gastroenterol Hepatol. 2016;10:13–20.
Amarapurkar D, Dhorda M, Kirpalani A, Amarapurkar A, Kankonkar S. Prevalence of hepatitis C genotypes in Indian patients and their clinical significance. J Assoc Physicians India. 2001;49:983–5.
Maheshwari A, Thuluvath PJ. Management of acute hepatitis C. Clin Liver Dis. 2010;14:169–76.
Mukherjee R, Burns A, Rodden D, et al. Diagnosis and management of hepatitis C virus infection. J Lab Autom. 2015;20:519–38.
Villareal VA, Rodgers MA, Costello DA, Yang PL. Targeting host lipid synthesis and metabolism to inhibit dengue and hepatitis C viruses. Antivir Res. 2015;124:110–21.
Meissner EG, Lee YJ, Osinusi A, et al. Effect of sofosbuvir and ribavirin treatment on peripheral and hepatic lipid metabolism in chronic hepatitis C virus, genotype 1-infected patients. Hepatology. 2015;61:790–801.
Simon G, Butt A. Lipid dysregulation in hepatitis C virus, and impact of statin therapy upon clinical outcomes. World J Gastroenterol. 2015;21:8293–303.
Antonelli A, Ferrari SM, Giuggioli D, et al. Hepatitis C virus infection and type 1 and type 2 diabetes mellitus. World J Diabetes. 2014;5:586–600.
Recommendations for testing, managing, and treating hepatitis C [Internet]: American Association for the Study of Liver Diseases and Infectious Disease Society of America; 2016. [Cited 2016 July 25] Available from: http://hcvguidelines.org/sites/default/files/HCV-Guidance_July_2016_b.pdf
Kirby BJ, Symonds WT, Kearney BP, Mathias AA. Pharmacokinetic, pharmacodynamic, and drug-interaction profile of the hepatitis C virus NS5B polymerase inhibitor sofosbuvir. Clin Pharmacokinet. 2015;54:677–90.
Smith MA, Regal RE, Mohammad RA. Daclatasvir: a NS5A replication complex inhibitor for hepatitis C infection. Ann Pharmacother. 2016;50:39–46.
Jung HJ, Kim YS, Kim SG, et al. The impact of pegylated interferon and ribavirin combination treatment on lipid metabolism and insulin resistance in chronic hepatitis C patients. Clin Mol Hepatol. 2014;20:38–46.
Kuo YH, Chuang TW, Hung CH, et al. Reversal of hypolipidemia in chronic hepatitis C patients after successful antiviral therapy. J Formos Med Assoc. 2011;110:363–71.
Corey KE, Kane E, Munroe C, Barlow LL, Zheng H, Chung RT. Hepatitis C virus infection and its clearance alter circulating lipids: implications for long-term follow-up. Hepatology. 2009;50:1030–7.
Arain SQ, Talpur FN. Channa NA. A comparative study of serum lipid contents in pre and post IFN-alpha treated acute hepatitis C patients. Lipids Health Dis. 2015;14:117.
Endo D, Satoh K, Shimada N, Hokari A, Aizawa Y. Impact of interferon-free antivirus therapy on lipid profiles in patients with chronic hepatitis C genotype 1b. World J Gastroenterol. 2017;23:2355–64.
Hashimoto S, Yatsuhashi H, Abiru S, et al. Rapid increase in serum low-density lipoprotein cholesterol concentration during hepatitis C interferon-free treatment. PLoS One. 2016;11:e0163644.
Townsend K, Meissner EG, Sidharthan S, Sampson M, Remaley AT, Tang L. Interferon-free treatment of hepatitis C virus in HIV/hepatitis C virus-coinfected subjects results in increased serum low-density lipoprotein concentration. AIDS Res Hum Retrovir. 2016;32:456–62.
Lawitz E, Mangia A, Wyles D, et al. Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med. 2013;368:1878–87.
ATP III guidelines at- a- glance quick reference (Internet). Texas: National Institutes of Health; 2001(Cited 2018 Feb 9). Available from https://www.nhlbi.nih.gov/files/docs/guidelines/atglance.pdf.
Younossi Z, Henry L. Systematic review: patient-reported outcomes in chronic hepatitis C - the impact of liver disease and new treatment regimens. Aliment Pharmacol Ther. 2015;41:497–520.
Ghany MG, Nelson DR, Strader DB, Thomas DL, Seeff LB. An update on treatment of genotype 1 chronic hepatitis C virus infection: 2011 practice guideline by the American Association for the Study of Liver Diseases. Hepatology. 2011;54:1433–44.
Nelson DR, Cooper JN, Lalezari JP, et al. All-oral 12-week treatment with daclatasvir plus sofosbuvir in patients with hepatitis C virus genotype 3 infection: ALLY-3 phase III study. Hepatology. 2015;61:1127–35.
Funding
None.
Author information
Authors and Affiliations
Contributions
A J: Involved in planning of study, collection, analysis and writing of draft of paper.
S C, B S: Involved in planning of the study, supervision of conduct of study and guided in writing the manuscript.
S S: Helped in clinical supervision of the data collection and review of manuscript.
Corresponding author
Ethics declarations
Conflict of interest
AJ, BSK, SS, and SC declare that they do not have any conflict of interest to report.
Ethical clearance
The authors declare that the study was performed in a manner conforming to the Helsinki declaration of 1975, as revised in 2000 and 2008 concerning human and animal rights, and the authors followed the policy concerning informed consent as shown on Springer. com.
Disclaimer
The authors are solely responsible for the data and the content of the paper. In no way, the Honorary Editor-in-Chief, Editorial Board Members, or the printer/publishers are responsible for the results/findings and content of this article.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jain, A., Kalra, B.S., Srivastava, S. et al. Effect of sofosbuvir and daclatasvir on lipid profile, glycemic control and quality of life index in chronic hepatitis C, genotype 3 patients. Indian J Gastroenterol 38, 39–43 (2019). https://doi.org/10.1007/s12664-019-00935-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12664-019-00935-w