Abstract
Purpose
Although a maxillary nerve (MN) block reportedly provides satisfactory analgesia for midface surgery and chronic maxillofacial pain syndromes, a safe and reliable MN block technique has not been reported. The goal of this anatomical study was to quantify the various angles and depth of the block needle, as well as to evaluate the impact of volume on the extent of injectate spread that might influence anesthetic coverage and block-related complications.
Methods
Following an ultrasound-guided suprazygomatic MN block with dye injection, a dissection was performed in the pterygopalatine fossa (PPF) of four lightly embalmed cadaveric specimens. Half of the specimens were injected with 5 mL of dye, and the other half with 1 mL of dye. The needle depth was measured from the ultrasound images and using rubber markers. Following injection, dissection was performed to map the area of dye spread.
Results
The median [interquartile range (IQR)] distance from the skin to the PPF was 37 [36–43] mm and 47 [40–50] mm by ultrasound and rubber marker methods, respectively. The median [IQR] needle orientation was 14 [11–32] degrees inferiorly and 15 [10–17] degrees posteriorly. The PPF was consistently dyed in the 5 mL group, but sporadically dyed in the 1 mL group. In the 5 mL group, spread outside of the PPF was seen.
Conclusions
We showed that 5 mL of injectate far exceeds the capacity of the PPF, leading to drug spread outside of the PPF. Moreover, we found that 1 mL of injectate largely covered the nerve, suggesting a more efficacious and safer block procedure. This finding will need confirmation in future clinical studies.
Résumé
Objectif
Bien qu’un bloc du nerf maxillaire semblerait fournir une analgésie satisfaisante pour une chirurgie de la partie moyenne du visage et pour les syndromes de douleur maxillo-faciale chronique, aucune technique de bloc du nerf maxillaire sécuritaire et fiable n’a encore été rapportée. L’objectif de cette étude anatomique était de quantifier les divers angles et la profondeur de l’aiguille pour le bloc, ainsi que d’évaluer l’impact du volume sur l’étendue de la diffusion du produit injecté qui pourrait influencer la couverture anesthésique et les complications liées au bloc.
Méthode
À la suite d’un bloc du nerf maxillaire supra-zygomatique échoguidé contenant une injection de colorant, une dissection a été réalisée dans la fosse ptérygo-maxillaire sur quatre spécimens cadavériques légèrement embaumés. La moitié des spécimens a reçu une injection de 5 mL de colorant, et l’autre moitié a reçu une injection de 1 mL de colorant. La profondeur de l’aiguille a été mesurée sur les images échographiques et avec des marqueurs en caoutchouc. Après l’injection, une dissection a été réalisée afin de déterminer la zone de diffusion du colorant.
Résultats
La distance médiane [écart interquartile (ÉIQ)] entre la peau et la fosse ptérygo-maxillaire était de 37 [36–43] mm et de 47 [40-50] mm selon la méthode par échographie et celle de marquage, respectivement. L’orientation médiane [ÉIQ] de l’aiguille était de 14 [11-32] degrés en inférieur et de 15 [10–17] degrés en postérieur. La fosse ptérygo-maxillaire était teintée de manière uniforme dans le groupe 5 mL, mais de façon sporadique dans le groupe 1 mL. Dans le groupe 5 mL, une diffusion hors de la fosse ptérygo-maxillaire a été observée.
Conclusion
Nous avons démontré qu’une injection de 5 mL excédait la capacité de la fosse ptérygo-maxillaire, entraînant une diffusion du médicament hors de cette zone. En outre, nous avons observé qu’une injection de 1 mL couvrait largement le nerf, ce qui suggère un bloc efficace et plus sécuritaire. Ces résultats devront être confirmés dans d’autres études cliniques à l’avenir.
Similar content being viewed by others
The maxillary nerve (MN) is a purely sensory nerve that supplies innervation to the lower eyelid, upper lip, cheek, upper dental arch, maxillary sinus, hard and soft palate, posterior nasal cavity, and nasal ala. A MN block was first described in the early 20th century for dental purposes.1 Although the MN block has been traditionally used for the diagnosis and treatment of chronic oral and maxillofacial pain syndromes, it has recently been re-appraised for various indications, such as cleft palate surgery, oral and orthognathic surgery, and maxillary bone fractures.2,3,4,5,6,7,8,9
Maxillofacial surgery can be associated with a risk of postoperative upper airway obstruction and related respiratory complications. Opiate use may further contribute to the development and exacerbation of these complications.10,11,12 Regional anesthesia for orthognathic and maxillofacial surgery has been reported to reduce intraoperative stress responses and perioperative opioid consumption, providing an adjunctive anesthetic technique with an overall improved safety profile.13,14
Numerous complications are associated with the MN block, including persistent paresthesia, hematoma formation, diplopia, transient ophthalmoplegia and ptosis, penetration of the orbit, temporal blindness, and brainstem anesthesia.15,16,17,18 Among all types of approach, the suprazygomatic approach has been reported to have a more favourable safety profile.2,4,19,21 While some of the complications are related to the particular anatomical approach used, others are related to the volume of anesthetic injected into the pterygopalatine fossa (PPF). The average volume of the PPF in adults has been reported from studies in dry skulls as close to one mL.22,23 Nevertheless, two to five mL is typically injected when clinically performing this block.4,5,7,22,24 Consequently, the excess quantity of local anesthetic may transit through the infratemporal fossa, spreading intracranially, or into the orbit. For this reason, it has been suggested to inject a smaller volume of local anesthetic to improve block safety.22,23
There is no consensus about the minimum volume needed to perform a safe and reliable MN block, and no anatomical validation studies of the spread of anesthetic in the PPF have been published. The purpose of this study was to compare different injectate volumes to study the sites and the extent of drug spread, as well as to quantify the various angles and depth of the block needle relative to the skin when performing an ultrasound-guided MN block.
Methods
This project was approved by the University of Toronto, Health Sciences Research Ethics Board (17 January 2017; protocol reference # 33958). Maxillary nerve dye injection and dissection were performed in the PPF of four lightly embalmed cadaveric specimens. Lightly embalmed specimens have been reported to have similar tissue qualities to those of live subjects with minimal structural distortion.25,26,27 All specimens were legally obtained and stored in the Department of Anatomy at the University of Toronto. Donors consented that after death their bodies could be used at the Division of Anatomy for education and research purposes. Specimens were excluded if there was any sign of pathology, injury, or previous surgery of the head.
Block target and technique
The MN exits the skull through the foramen rotundum, passing through the PPF before entering the inferior orbital fissure, which the nerve traverses anteriorly to finally reach the midface via the infraorbital foramen.2,3,19,28 The PPF is an inverted pyramid-shaped space located inferior to the orbital apex, and bounded by the junction of maxilla, sphenoid, and palatine bones. The PPF communicates with the nasal and oral cavities and the orbit. Important structures are located in the PPF including the distal branches of the maxillary artery, emissary veins, lymphatics and nerves, as well as the pterygopalatine ganglion, MN and its branches, and the vidian nerve29,30,31 (Figs 1 and 2).
This image depicts the infratemporal fossa. The mandibular ramus, condyle, zygomatic arch, and lateral pterygoid muscle have been removed. The denudated lateral pterygoid plate, the internal maxillary artery, and inferior alveolar nerve can be seen. The facial skin has been reflected forward, tenting the infraorbital nerve into view. The location of the pterygopalatine ganglion, and the maxillary nerve and its branches are projected onto the surface bony anatomy in yellow. The pixelated box is located at the level of the orbital cavity
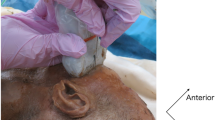
The anatomic specimens were placed in a stabilization device to prevent movement during the injection. All injections were performed by the same anesthetist (GE), using an ultrasound-guided suprazygomatic approach. A portable ultrasound unit with an 8–13 MHz linear array probe was utilized (GE Venue 40, Wauwatosa, WI, USA). Similar to the technique used by Sola et al.,20 the ultrasound transducer was placed in the infrazygomatic area, with an inclination of 45º in the transverse plane (Fig. 3A). A 22G 90-mm Quincke-point spinal needle (Spinocan; Becton Dickinson, Franklin Lakes, NJ, USA) was used for the injection. The needle was inserted perpendicular to the skin at the frontozygomatic angle and advanced to the greater wing of the sphenoid (Fig. 3B). The needle was then redirected and advanced to the PPF (Figs 3A and 3C). After needle placement, one or five mL of the dye was injected in a randomized order (Fig. 3D). An aqueous dye (0.5 mL of methylene blue mixed in 19.5 mL of water) of approximately equal viscosity to local anesthetic was injected. One-half of the specimens were injected with 5 mL of dye, representing the volume traditionally used for the MN block, and the other half were injected with 1 mL of dye, representing the previously calculated volume of the PPF.
Ultrasound images showing the location of the ultrasound probe on the face and needle entry point (A), the distance from the entry point of the needle at the frontozygomatic angle to the greater wing of the sphenoid (B) and the pterygopalatine fossa (C). Dye spread is shown in D (arrows). PPF = pterygopalatine fossa
Fifteen minutes post-injection, the needle was lightly tapped with a mallet to anchor its tip into the nasal bones thus preventing displacement. High quality digital photographs were taken from anterior and superior view. The needle depth was measured from the ultrasound pictures and also by using a rubber marker at the level of the skin. The needle angles were measured with a digital protractor (ImageMeter, version 2.22.1. Dirk Farin) by two independent investigators. Similar to the method used by Prigge et al.,21 the plane perpendicular to the median plane was considered to be 0° (utilizing the anterior and superior view). If the needle was angled superiorly (in the anterior view) or anteriorly (in the superior view) towards the PPF, it was considered an increase (+) in the angle. Any inferior or posterior angling of the needle was considered a decrease (-) in the angle (Fig. 4).
We then performed a cadaveric dissection involving a layer by layer removal of the soft tissue and bony structures overlying the infratemporal fossa. The facial skin, the parotid gland, the zygoma and attached masseter, the temporalis fascia and attached coronoid process, and the condyle and attached lateral pterygoid muscle were each isolated and removed by a combination of soft tissue dissection and osteotomies. This facilitated a clear exposure of the infratemporal fossa and its contents, and access to the PPF. The entrance to the PPF, the pterygomaxillary fissure, the surrounding lateral pterygoid plate, and the posterior maxilla were carefully dissected. Throughout the dissection, a systematic note was made of the various structures that had been stained by the dye. Following this, the posterior maxillary bone was dissected and removed to gain wider access to the PPF. The course of the second division of the trigeminal nerve was also delineated through the floor of the orbit and the orbital contents were removed. Its course was delineated to the level of foramen rotundum, as well as the intracranial course of the trigeminal nerve. This dissection allowed a full view of the area of the pterygopalatine ganglion. High-quality photographs were taken serially throughout the dissection.
Using the collected images and markers, we calculated the following metrics for comparison. From the ultrasound images, we recorded the i) distance from the entry point of the needle at the frontozygomatic angle to the greater wing of the sphenoid (skin to greater wing of the sphenoid; S-GWS); ii) distance from the entry point of the needle at the frontozygomatic angle to the PPF (skin to pterygopalatine fossa; S-PPF); and iii) the dye spread. Using the surface markers and views, we recorded i) the needle angles from the anterior and superior view, and ii) the distance from the entry point of the needle at the frontozygomatic angle to the PPF using the rubber markers. From the dissection images, we noted i) the area of intra/extra PPF dye spread; and ii) the frequency of capture of each nerve and pterygopalatine ganglion with 1- and 5-mL injection volumes.
Descriptive statistics was analyzed using SPSS 23.0 statistical package.
Results
We obtained four lightly embalmed cadaveric specimens from two females (84 and 70 yr of age). The ultrasound images were easily obtained and were high quality, allowing for accurate observation of dye spread and needle movements. By ultrasound, the median [interquartile range (IQR)] distance from the skin to the greater wing of the sphenoid and to the PPF was 13 [11–16] mm and 37 [36–43] mm, respectively. The median [IQR] observed distance from the frontozygomatic angle to the PPF by the rubber marker method was 47 [40–50] mm. The median [IQR] needle orientation was 14 [11–32] degrees inferiorly and 15 [10–17] degrees posteriorly (Table). In one specimen, the needle entrance to the PPF was difficult to negotiate because of a small pterygopalatine fissure, and the needle was angulated more caudally (38º). Dye spread into the orbit, cranial fossa, or nasal cavity was not observed in any experiment, even when the higher volume (5 mL) of dye was injected. Nevertheless, in the high-volume group, we did observe spread back into the infratemporal fossa and above the temporal muscle, reaching to the deep temporal nerves, masseteric nerves, and the mandibular nerve branches (i.e., lingual and inferior alveolar nerves). The PPF, pterygopalatine ganglion, infraorbital nerve, and palatine nerves were dyed consistently in the 5 mL group, but only sporadically in the 1 mL group (Table and Fig. 5).
Serial dissection showing dye spread following 1 mL (A–D) and 5 mL (E–H) injections. Dissection from superficial (top) to deep (bottom) planes. DtN = deep temporal nerve; LN = lingual nerve; IaN = inferior alveolar nerve; MsN = masseteric nerve. D and H show the entrance to the pterygopalatine fossa (pterygomaxillary fissure). PmF = pterygomaxillary fissure; PsM = posterior surface of maxilla; PtP = pterygoid process
Discussion
This is the first anatomical validation reporting the distance from the frontozygomatic angle to the PPF, as well as the needle angulations and dye spread when performing a MN block via the suprazygomatic approach.
We found that the median [IQR] distance from the skin to the greater wing of the sphenoid was 13 [11–16] mm by ultrasound imaging, and the median [IQR] distance from the skin to the PPF was 37 [36–43] mm and 47 [40–50] mm using ultrasound imaging and the rubber marker method, respectively. These values likely differ because the ultrasound image was obtained from the infrazygomatic area, while the rubber marker utilized the needle direction from the frontozygomatic angle. Furthermore, pressure needs to be applied on the ultrasound probe, which compresses the tissue, potentially shortening the distance.
Utilizing computed tomography scans, Captier et al.19 reported greater mean (standard deviation) distances from the skin to the greater wing of the sphenoid and to the foramen rotundum in infants [24 (3) mm and 47 (4) mm, respectively], than our findings in adult patients. These differences illustrate how developmental variation in the size and shape of the skull and in the proportion of soft tissue can affect the distance measurements and produce potentially unexpected variations in the MN block procedure.
Prigge et al.21 found that the distance from the frontozygomatic angle to the PPF was 21 mm and 22 mm in neonates, for cadaveric specimens and dry skulls, respectively, whereas in infant cadaveric specimens this distance was 27 mm, highlighting the importance of developmental variation and tissue proportion in determining a safe insertion distance.
The equivalent needle insertion distances have not been objectively reported in adults. As a surrogate for distance measurement, Stajcic et al.4 utilized a 20G, 80-mm spinal needle, fitted with a rubber marker 50-mm from the tip. Unfortunately, the authors did not report the distance from the skin to the PPF, making comparison of their results with ours impossible. Similarly, Bouzinac et al.24 used a 50-mm needle to perform ultrasound-guided MN blocks by a suprazygomatic approach in adults. They also did not report the distance from the skin to the PPF.
Following the findings of our study, when performing a MN block, the needle should be advanced an average of 14º inferiorly and 15º posteriorly, which is consistent with the results published by Prigge et al. They found that the needle should be advanced between 8° and 15° in a posterior direction. In contrast, our findings are different than those previously published in adults. Stajcic and Todorovic4 advanced the needle angulated 60º towards the sagittal and 10º towards the horizontal planes. Bouzinac et al.24 reported an ultrasound-guided MN block with the needle inserted at the frontozygomatic angle and oriented 45º to the skin. Similarly, according to Captier’s19 and Chiono’s studies in infants, the needle was directed from the frontozygomatic angle in a 20° forward and 10° downward direction. Although some of the variance in insertion angle can be attributed to the patient group under investigation (neonate, infant, or adult), the majority is associated with intrinsic anatomical differences in the population and the measuring technique. Related to the disparities in reported angle and approach, there is a high likelihood that, unless directly visualized, the needle tip in many studies is not actually in the PPF and block efficacy is obtained by injecting a larger volume of anesthetic. Therefore, the standardization of approach angle and angle measurement should lead to a more consistent and safe use of the MN block by ensuring that the needle tip placement is in the PPF, thus allowing for better nerve targeting and a smaller volume of local anesthetic to be injected. This would also facilitate clinical investigation as studies could be directly compared more easily.
It is likely that many of the reported MN block complications are directly related to the volume of local anesthetic used. The mean volume of the PPF in adults has been reported to be between 0.722 and 1.2 mL23 and it was not influenced by the cephalic and upper facial indexes. Nevertheless, the usual reported quantity of local anesthetic injected (2–5 mL) greatly exceeds the volume of the PPF, and the excess quantity could transit the infratemporal fossa, or enter either the infraorbital canal or the middle cranial fossa through the foramen rotundum.23
We found that injecting 5 mL of dye ensured consistent bathing of the MN nerve, but a significant amount of dye spread into the infratemporal fossa and above the temporalis muscle, blocking even the mandibular nerve branches, masseteric nerves, and deep temporal nerves. In contrast, when we injected 1 mL, there was minimal to no spread of dye into the infratemporal fossa, even though the MN was less consistently fully covered. Nevertheless, this dye coverage does not directly correlate with local anesthesia diffusion into the nerve and with block efficacy. Our findings indicate that 5 mL of injectate is well beyond the volume capacity of the PPF and leads to non-specific blockage of anatomically close nerves, with potential adverse side effects, and that a volume slightly over 1 mL might be needed to ensure efficacious analgesia.
In one specimen, we found it challenging to negotiate the needle entrance into the PPF and the needle was angulated more caudally. Similarly, Stajcic et al.22 found samples with either an enlarged sphenoidal process (15% of skulls) or a narrow pterygomaxillary fissure (< 2 mm in 8%), thus preventing the entrance of the needle tip to the pterygopalatine fissure. The difficulties arising from this natural anatomic variation should be considered when attempting a MN block.
The suprazygomatic approach at the level of the suprazygomatic angle has been proposed as the safest, easiest, and most reliable of all recommended approaches to the PPF. In theory, this technique prevents penetration of the base of the skull and the orbit4 and the bony landmarks are more superficial and more easily palpated, which simplifies block performance and improves safety.21 Using this approach, we did not find dye spread into the orbit or into the intracranial fossa, even with a higher volume of injectate, illustrating an enhanced block safety.
In conclusion, our findings suggest that the ultrasound-guided suprazygomatic approach could be a safe approach to perform a MN block. In adult patients, the needle should be placed at the frontozygomatic angle and advanced in a postero-inferior direction (15º and 14º, respectively) for approximately 37 mm. While 5 mL of local anesthesia injectate is common, this volume far exceeds the capacity of the PPF and could lead to inadvertent drug spread and non-specific nerve blockage. That said, we did not observe fluid migration into the most significant off-target sites (i.e., the orbit and cranial fossa) with volumes up to 5 mL. We found that 1 mL of injectate did not spread out of the PPF, and that this volume largely covered the nerve, likely leading to an efficacious and safer block procedure. Our findings can be used to improve MN block safety and efficacy. To better account for anatomic variation, more precisely determine the needed local anesthesia volume, and more accurately delineate block safety and feasibility, studies with a larger sample size are needed.
References
Aoun G, Zaarour I, Sokhn S, Nasseh I. Maxillary nerve block via the greater palatine canal: an old technique revisited. J Int Soc Prev Community Dent 2015; 5: 359-64.
Mesnil M, Dadure C, Captier G, et al. A new approach for perioperative analgesia of cleft palate repair in infants: the bilateral suprazygomatic maxillary nerve block. Pediatr Anesth 2010; 20: 343-9.
Chiono J, Raux O, Bringuier S, et al. Bilateral suprazygomatic maxillary nerve block for cleft palate repair in children: a prospective, randomized, double-blind study versus placebo. Anesthesiology 2014; 120: 1362-9.
Stajcic Z, Todorovic L. Blocks of the foramen rotundum and the oval foramen: a reappraisal of extraoral maxillary and mandibular nerve injections. Br J Oral Maxillo Surg 1997; 35: 328-33.
Robiony M, Demitri V, Costa F, Politi M, Cugini U. Truncal anaesthesia of the maxillary nerve for outpatient surgically assisted rapid maxillary expansion. Br J Oral Maxillofac Surg 1998; 36: 389-91.
Kohase H, Miyamoto T, Umino M. A new method of continuous maxillary nerve block with an indwelling catheter. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2002; 94: 162-6.
Thangavelu K, Kumar NS, Kannan R, Arunkumar J, Rethish E. Maxillary nerve block in management of maxillary bone fractures: our experience. Anesth Essays Res 2012; 6: 58-61.
Radder K, Shah A, Fatima S, Kothari C, Zakaullah S, Siddiqua A. Efficacy and feasibility of frontozygomatic angle approach for extra oral maxillary nerve block in oral surgery: a descriptive clinical trial. J Maxillofac Oral Surg 2014; 13: 231-7.
Geier KO. Maxillary nerve block for zygoma and orbital floor fractures reduction (Portuguese). Rev Bras Anestesiol 2003; 53: 512-7.
Aziz SR, Agnihotri N, Ziccardi VB. Lobar collapse immediately after orthognathic surgery. J Oral Maxillofac Surg 2010; 68: 2335-8.
Chebel NA, Ziade D, Achkouty R. Bilateral pneumothorax and pneumomediastinum after treatment with continuous positive airway pressure after orthognathic surgery. Br J Oral Maxillofac Surg 2010; 48: e14-5.
Kim YK. Complications associated with orthognathic surgery. J Assoc Oral Maxillofac Surg 2017; 43: 3-15.
Mamiya H, Ichinohe T, Kaneko Y. Effects of block analgesia on attenuating intraoperative stress responses during oral surgery. Anesth Prog 1997; 44: 101-5.
Noma T, Ichinohe T, Kaneko Y. Inhibition of physiologic stress responses by regional nerve block during orthognathic surgery under hypotensive anesthesia. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1998; 86: 511-5.
Nish IA, Pynn BR, Holmes HI, Young ER. Maxillary nerve block: a case report and review of the intraoral technique. J Can Dent Assoc 1995; 61: 305-10.
Malamed SF, Trieger N. Intraoral maxillary nerve block: an anatomical and clinical study. Anesth Prog 1983; 30: 44-8.
Sved AM, Wong JD, Donkor P, et al. Complications associated with maxillary nerve block anaesthesia via the greater palatine canal. Aust Dent J 1992; 37: 340-5.
Nique TA, Bennett CR. Inadvertent brainstem anesthesia following extraoral trigeminal V2-V3 blocks. Oral Surg Oral Med Oral Pathol 1981; 51: 468-70.
Captier G, Dadure C, Leboucq N, Sagintaah M, Canaud N. Anatomic study using three-dimensional computed tomographic scan measurement for truncal maxillary nerve blocks via the suprazygomatic route in infants. J Craniofacial Surg 2009; 20: 224-8.
Sola C, Raux O, Savath L, Macq C, Capdevila X, Dadure C. Ultrasound guidance characteristics and efficiency of suprazygomatic maxillary nerve blocks in infants: a descriptive prospective study. Pediatr Anesth 2012; 22: 841-6.
Prigge L, van Schoor AN, Bosman MC, Bosenberg AT. Clinical anatomy of the maxillary nerve block in pediatric patients. Pediatr Anesth 2014; 24: 1120-6.
Stajcic LS, Gacic B, Popovic N, Stajcic Z. Anatomical study of the pterygopalatine fossa pertinent to the maxillary nerve block at the foramen rotundum. Int J Oral Maxillofac Surg 2010; 39: 493-6.
Gallardo C, Andrés C, Galdames S, et al. Relationship between pterygopalatine fossa volume and cephalic and upper facial indexes. Int J Morphol 2008; 26: 393-6.
Bouzinac A, Tournier JJ, Dao M, Delbos A. Ultrasound-guided maxillary nerve block in adults: feasibility and efficiency for postoperative analgesia after maxillary osteotomy. Minerva Anestesiol 2014; 80: 860-1.
Tsui BC, Dillane D, Pillay J, Ramji AK, Walji AH. Cadaveric ultrasound imaging for training in ultrasound-guided peripheral nerve blocks: lower extremity. Can J Anesth 2007; 54: 475-80.
Anderson SD. Practical light embalming technique for use in the surgical fresh tissue dissection laboratory. Clin Anat 2006; 19: 8-11.
Coleman R, Kogan I. An improved low-formaldehyde embalming fluid to preserve cadavers for anatomy teaching. J Anat 1998; 192(Pt 3): 443-6.
Rodella LF, Buffoli B, Labanca M, Rezzani R. A review of the mandibular and maxillary nerve supplies and their clinical relevance. Arch Oral Biol 2012; 57: 323-34.
Tashi S, Purohit BS, Becker M, Mundada P. The pterygopalatine fossa: imaging anatomy, communications, and pathology revisited. Insights Imaging 2016; 7: 589-99.
Choi J, Park HS. The clinical anatomy of the maxillary artery in the pterygopalatine fossa. J Oral Maxillofac Surg 2003; 61: 72-8.
Oomen KP, Pameijer FA, Zwanenburg JJ, Hordijk GJ, De Ru JA, Bleys RL. Improved depiction of pterygopalatine fossa anatomy using ultrahigh-resolution magnetic resonance imaging at 7 tesla. ScientificWorldJournal. 2012; DOI: https://doi.org/10.1100/2012/691095.
Conflicts of interest
None declared.
Editorial responsibility
This submission was handled by Dr. Hilary P. Grocott, Editor-in-Chief, Canadian Journal of Anesthesia.
Author contributions
Gastón Echaniz helped conceive the study idea, design the study, perform the dye injection and cadaveric dissection, collect and analyze the data, design figures, and write the manuscript. Vincent Chan helped design the study and write the manuscript. Jason Maynes helped design the study, analyze the data, and write the manuscript. Yelda Jozaghi helped with cadaveric dissection and to write the manuscript. Anne Agur helped conceive the study idea, design the study, write the manuscript, and design the figures.
Financial sources
Support was provided solely from institutional and/or departmental sources.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The work is to be attributed to Department of Surgery Vall d’Hebron Hospital, Universitat Autònoma de Barcelona, Barcelona, Spain.
Rights and permissions
About this article
Cite this article
Echaniz, G., Chan, V., Maynes, J.T. et al. Ultrasound-guided maxillary nerve block: an anatomical study using the suprazygomatic approach. Can J Anesth/J Can Anesth 67, 186–193 (2020). https://doi.org/10.1007/s12630-019-01481-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12630-019-01481-x