Abstract
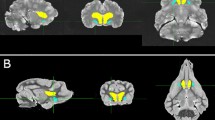
The degu (Octodon degus) is a rodent that normally constructs burrows for nesting and rearing. To navigate inside these burrows, degus may use idiothetic and/or sensory cues more than visual information, which is less effective in burrows. Spatial information for navigation is processed in several key brain regions including the retrosplenial cortex (RS). However, the structural characteristics of the degu RS have not been previously reported. The present study measured the sizes of the RS and constituent areas 29 and 30 in the degu, and compared these to those found in the rat, which is a terrestrial rodent. The proportion of the rostrocaudal length of the entire RS relative to that of the entire cortex was significantly larger in degus versus rats. The proportion of the rostrocaudal length of the RS at levels rostral to the splenium of the corpus callosum relative to that of the entire cortex was also significantly larger in degus versus rats. Furthermore, the ratio of the estimated volume of area 29 relative to that of area 30 was significantly larger in degus versus rats. These results show that the degu has a rostrocaudally longer rostral RS with a larger area 29 compared to the rat, which suggests that these structural features may be relevant to differences in spatial information processing between the fossorial degu and terrestrial rat.







Similar content being viewed by others
Abbreviations
- cc:
-
Corpus callosum
- c-RS:
-
Caudal retrosplenial cortex
- Cx:
-
Cortex
- Ent:
-
Entorhinal cortex
- L:
-
Layer
- P:
-
Parietal cortex
- Pre:
-
Presubiculum
- r-RS:
-
Rostral retrosplenial cortex
- RS:
-
Retrosplenial cortex
- Sub:
-
Subiculum
- V:
-
Visual cortex
- 29:
-
Area 29
- 30:
-
Area 30
References
Brotons-Mas JR, Schaffelhofer S, Guger C, O’Mara SM, Sanchez-Vives MV (2017) Heterogeneous spatial representation by different subpopulations of neurons in the subiculum. Neuroscience 343:174–189
Chen LL, Lin L-H, Green EJ, Barnes CA, McNaughton BL (1994) Head-direction cells in the rat posterior cortex. I. Anatomical distribution and behavioral modulation. Exp Brain Res 101:8–23
Colby LA, Rush HG, Mahoney MM, Lee TM (2012) Degu. In: Suckow MA, Stevens KA, Wilson RP (eds) The laboratory rabbit, guinea pig, hamster, and other rodents. Elsevier, London, pp 1032–1054
Colonnello V, Iacobucci P, Fuchs T, Newberry RC, Panksepp J (2011) Octodon degus. A useful animal model for social-affective neuroscience research: basic description of separate distress, social attachments and play. Neurosci Biobehav Rev 35:1854–1863
Ebensperger LA, Bozinovic F (2000) Communal borrowing in the hystricognath rodent, Octodon degus: a benefit of sociality? Behav Ecol Sociobiol 47:365–369
Finch DM, Derian EL, Babb TL (1984) Afferent fibers to rat cingulate cortex. Exp Neurol 83:468–485
Fulk GW (1976) Notes on the activity, reproduction, and social behavior of Octodon degus. J Mammal 57:495–505
Hedrick BP, Dickson BV, Dumont ER, Pierce SE (2020) The evolutionary diversity of locomotor innovation in rodents is not linked to proximal limb morphology. Sci Rep 10:717
Heidbreder CA, Groenewegen HJ (2003) The medial prefrontal cortex in the rat: evidence for a dorso-ventral distinction based upon functional and anatomical characteristics. Neurosci Biobehav Rev 27:555–579
Honda Y, Ishizuka N (2015) Topographic distribution of cortical projection cells in the rat subiculum. Neurosci Res 92:1–20
Hoover WB, Vertes RP (2007) Anatomical analysis of afferent projections to the medial prefrontal cortex in the rat. Brain Struct Funct 212:149–179
Hoover WB, Vertes RP (2011) Projections of the medial orbital and ventral orbital cortex in the rat. J Comp Neurol 519:3766–3801
Jeki V, Hauptman K, Knotek Z (2011) Diseases in pet degus: a retrospective study in 300 animals. J Small Anim Prac 52:107–112
Jones BF, Groenewegen HJ, Witter MP (2005) Intrinsic connections of the cingulate cortex in the rat suggest the existence of multiple functionally segregated networks. Neuroscience 133:193–207
Kádár E, Vico-Varela E, Aldavert-Vera L, Huguet G, Morgado-Bernal I, Segura-Torres P (2019) Increase in c-Fos and Arc protein in retrosplenial cortex after memory-improving lateral hypothalamic electrical stimulation treatment. Neurobiol Learn Mem 128:117–124
Kumazawa-Manita N, Hashikawa T, Iriki A (2018) The 3D stereotaxic brain atlas of the degu. Springer Japan, Tokyo
Lee T, Alloway KD, Kim U (2011) Interconnected cortical networks between primary somatosensory cortex septal columns and posterior parietal cortex in rat. J Comp Neurol 519:405–419
Milczarek MM, Vann SD, Sengpiel F (2018) Spatial memory engram in the mouse retrosplenial cortex. Curr Biol 28:1975–1980
Miyashita T, Rockland KS (2007) GABAergic projections from the hippocampus to the retrosplenial cortex in the rat. Eur J Neurosci 26:1193–1204
Naber PA, Witter MP (1998) Subicular efferents are organized mostly as parallel projections: a double-labeling, retrograde-tracing study in the rat. J Comp Neurol 393:284–297
Nelson AJD, Perry JC, Vann SD (2018) The Papez circuit and recognition memory: contributions of the medial diencephalon and retrosplenial cortex to what, where and when aspects of object recognition memory. In: Ennaceur A, De Souza Silva MA (eds) Handbook of object novelty recognition. Elsevier, London, pp 217–226
Olsen GM, Ohara S, Iijima T, Witter MP (2017) Parahippocampal and retrosplenial connections of rat posterior parietal cortex. Hippocampus 27:335–358
Pérez MJ, Cassini GH, Díaz MM (2021) The forelimbs of Octodonidae (Rodentia: Mammalia): substrate use, morphology, and phylogenetic signal. Zoology 144:125879
Pothuizen HHJ, Davies M, Albasser MM, Aggleton JP, Vann SD (2009) Granular and dysgranular retrosplenial cortices provide quantitatively different connections to spatial working memory: evidence from immediate-early gene imaging in rats. Eur J Neurosci 30:877–888
Pothuizen HHJ, Davies M, Aggleton JP, Vann SD (2010) Effects of selective granular retrosplenial cortex lesions on spatial working memory in rats. Behav Brain Res 208:566–575
Samuels JX, Van Valkenburgh BV (2008) Skeletal indicators of locomotor adaptations in living and extinct rodents. J Morphol 269:1387–1411
Shibata H (1994) Terminal distribution of projections from the retrosplenial area to the retrohippocampal region in the rat, as studied by anterograde transport of biotinylated dextran amine. Neurosci Res 20:331–336
Shibata H, Naito J (2008) Organization of anterior cingulate and frontal cortical projections to the retrosplenial cortex in the rat. J Comp Neurol 506:30–45
Shibata H, Kondo S, Naito J (2004) Organization of retrosplenial cortical projections to the anterior cingulate, motor, and prefrontal cortices in the rat. Neurosci Res 49:1–11
Shibata H, Honda Y, Sasaki H, Naito J (2009) Organization of intrinsic connections of the retrosplenial cortex in the rat. Anat Sci Int 84:280–292
Sigwald EL, Bignante EA, De Olmos S, Lorenzo A (2019) Fear-context association during memory retrieval requires input from granular to dysgranular retrosplenial cortex. Neurobiol Learn Mem 163:107036
Sigwald EL, De Olmos S, Lorenzo A (2020) Retrograde and anterograde contextual fear amnesia induced by selective elimination of layer IV–Va neurons in the granular retrosplenial cortex (A29). Neurobiol Learn Mem 171:107229
Sul JH, Kim H, Huh N, Lee D, Jung MW (2010) Distinct roles of rodent orbitofrontal and medial prefrontal cortex in decision making. Neuron 66:449–460
Sul JH, Jo S, Lee D, Jung MW (2011) Role of rodent secondary motor cortex in value-based action selection. Nat Neurosci 14:1202–1208
Taube JS, Muller RU, Ranck JB Jr (1990) Head-direction cells recorded from the postsubiculum in freely moving rats. I Description and Quantitative Analysis. J Neurosci 10:420–435
Van Groen T, Wyss JM (1990a) Connections of the retrosplenial granular a cortex in the rat. J Comp Neurol 300:593–606
Van Groen T, Wyss JM (1990b) Extrinsic projections from area CA1 of the rat hippocampus: olfactory, cortical, and bilateral hippocampal formation projections. J Comp Neurol 302:515–528
Van Groen T, Wyss JM (1992) Connections of the retrosplenial dysgranular cortex in the rat. J Comp Neurol 315:200–216
Van Groen T, Wyss JM (2003) Connections of the retrosplenial granular b cortex in the rat. J Comp Neurol 463:249–263
Van Groen T, Kadish I, Wyss JM (2004) Retrosplenial cortex lesions of area Rgb (but not of area Rga) impair spatial learning and memory in the rat. Behav Brain Res 154:483–491
Vann SD, Aggleton JP (2002) Extensive cytotoxic lesions of the rat retrosplenial cortex reveal consistent deficits on tasks that tax allocentric spatial memory. Behav Neurosci 116:85–94
Vann SD, Aggleton JP (2005) Selective dysgranular retrosplenial cortex lesions in rats disrupt allocentric performance of the radial-arm maze task. Behav Neurosci 119:1682–1686
Vann SD, Aggleton JP, Maguire EA (2009) What does the retrosplenial cortex do? Nat Rev Neurosci 10:792–802
Vogt BA (2015) Cingulate cortex and pain architecture. In: Paxinos G (ed) The rat nervous system. Elsevier, Amsterdam, pp 575–599
Vogt BA, Miller MW (1983) Cortical connections between rat cingulate cortex and visual, motor, and postsubicular cortices. J Comp Neurol 216:192–210
Vogt BA, Paxinos G (2014) Cytoarchitecture of mouse and rat cingulate cortex with human homologies. Brain Struct Funct 219:185–192
Whishaw IQ, Maaswinkel H, Gonzalez CLR, Kolb B (2001) Deficits in allothetic and idiothetic behavior in rats with posterior cingulate cortex lesions. Behav Brain Res 118:67–76
Whitlock JR, Sutherland RJ, Witter MP, Moser M-B, Moser EI (2008) Navigating from hippocampus to parietal cortex. Proc Nat Acad Sci USA 105:14755–14762
Woods CA, Boraker DK (1975) Octodon degus. Mamm Spec 67:1–5
Acknowledgements
The study was supported by the annual funding (1306652) of the Tokyo University of Agriculture and Technology.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shibata, H., Kigata, T. Comparison of the retrosplenial cortex size between the degu (Octodon degus) and the Wistar rat (Rattus norvegicus). Anat Sci Int 98, 36–42 (2023). https://doi.org/10.1007/s12565-022-00669-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12565-022-00669-4