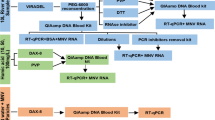
Abstract
The hollow fiber ultrafiltration (HFUF)-based microbial concentration method is widely applied for monitoring pathogenic viruses and microbial indicators in environmental water samples. However, the HFUF-based method can co-concentrate substances that interfere with downstream molecular processes—nucleic acid extraction, reverse transcription (RT), and PCR. These inhibitory substances are assumed to be hydrophobic and, therefore, expected to be excluded by a simple surfactant treatment before the silica membrane-based RNA extraction process. In this study, the efficacy and limitations of the sodium deoxycholate (SD) treatment were assessed by quantifying a process control and indigenous viruses using 42 surface water samples concentrated with HFUF. With some exceptions, which tended to be seen in samples with high turbidity (> 4.0 NTU), virus recovery by the ultrafiltration method was sufficiently high (> 10%). RNA extraction-RT-quantitative PCR (RT-qPCR) efficiency of the process control was insufficient (10%) for 30 of the 42 HFUF concentrates without any pretreatments, but it was markedly improved for 21 of the 30 inhibitory concentrates by the SD treatment. Detection rates of indigenous viruses were also improved and no substantial loss of viral RNA was observed. The SD treatment was particularly effective in mitigating RT-qPCR inhibition, although it was not effective in improving RNA extraction efficiency. The methodology is simple and easily applied. These findings indicate that SD treatment can be a good alternative to sample dilution, which is widely applied to mitigate the effect of RT-qPCR inhibition, and can be compatible with other countermeasures.



Similar content being viewed by others
Data Availability
The datasets generated during the current study are available from the corresponding author on reasonable request.
References
Boom, R., Sol, C. J., Salimans, M. M., Jansen, C. L., Wertheim-van Dillen, P. M., & van der Noordaa, J. (1990). Rapid and simple method for purification of nucleic acids. Journal of Clinical Microbiology, 28, 495–503.
Borgmästars, E., Jazi, M. M., Persson, S., Jansson, L., Rådström, P., Simonsson, M., Hedman, J., & Eriksson, R. (2017). Improved detection of norovirus and hepatitis A virus in surface water by applying pre-PCR processing. Food and Environmental Virology, 9, 395–405.
Bustin, S. A., Benes, V., Garson, J. A., Hellemans, J., Huggett, J., Kubista, M., Mueller, R., Nolan, T., Pfaffl, M. W., Shipley, G. L., Vandesompele, J., & Wittwer, C. T. (2009). The MIQE guidelines: Minimum information for publication of quantitative real-time PCR experiments. Clinical Chemistry, 55, 611–622.
Canh, V. D., Kasuga, I., Furumai, H., & Katayama, H. (2019). Viability RT-qPCR combined with sodium deoxycholate pre-treatment for selective quantification of infectious viruses in drinking water samples. Food and Environmental Virology, 11, 40–51.
Cashdollar, J. L., & Wymer, L. (2013). Methods for primary concentration of viruses from water samples: A review and meta-analysis of recent studies. Journal of Applied Microbiology, 115, 1–11.
Costafreda, M. I., Bosch, A., & Pintó, R. M. (2006). Development, evaluation, and standardization of a real-time TaqMan reverse transcription-PCR assay for quantification of hepatitis A virus in clinical and shellfish samples. Applied and Environmental Microbiology, 72, 3846–3855.
Coudray, C., Merle, G., Martin-Latil, S., Guillier, L., & Perelle, S. (2013). Comparison of two extraction methods for the detection of hepatitis A virus in lettuces using the murine norovirus as a process control. Journal of Virological Methods, 193, 96–102.
Dong, D., Yan, A., Liu, H., Zhang, X., & Xu, Y. (2006). Removal of humic substances from soil DNA using aluminium sulfate. Journal of Microbiological Methods, 66, 217–222.
D’Ugo, E., Marcheggiani, S., Fioramonti, I., Giuseppetti, R., Spurio, R., Helmi, K., Guillebault, D., Medlin, L. K., Simeonovski, I., Boots, B., Breitenbach, U., Koker, L., Albay, M., & Mancini, L. (2016). Detection of human enteric viruses in freshwater from European countries. Food and Environmental Virology, 8, 206–214.
Francy, D. S., Stelzer, E. A., Brady, A. M. G., Huitger, C., Bushon, R. N., Ip, H. S., Ware, M. W., Villegas, E. N., Gallardo, V., & Lindquist, H. D. A. (2013). Comparison of filters for concentrating microbial indicators and pathogens in lake water samples. Applied and Environmental Microbiology, 79, 1342–1352.
Gallardo, V. J., Morris, B. J., & Rhodes, E. R. (2019). The use of hollow fiber dialysis filters operated in axial flow mode for recovery of microorganisms in large volume water samples with high loadings of particulate matter. Journal of Microbiological Methods, 160, 143–153.
Gibson, K. E., & Schwab, K. J. (2011). Tangential-flow ultrafiltration with integrated inhibition detection for recovery of surrogates and human pathogens from large-volume source water and finished drinking water. Applied and Environmental Microbiology, 77, 385–391.
Haramoto, E., Katayama, H., Oguma, K., & Ohgaki, S. (2005). Application of cation-coated filter method to detection of noroviruses, enteroviruses, adenoviruses, and torque Teno viruses in the Tamagawa River in Japan. Applied and Environmental Microbiology, 71, 2403–2411.
Haramoto, E., Kitajima, M., Hata, A., Torrey, J. R., Masago, Y., Sano, D., & Katayama, H. (2018). A review on recent progress in the detection methods and prevalence of human enteric viruses in water. Water Research, 135, 168–186.
Haramoto, E., Kitajima, M., Kishida, N., Konno, Y., Katayama, H., Asami, M., & Akiba, M. (2013). Occurrence of pepper mild mottle virus in drinking water sources in Japan. Applied and Environmental Microbiology, 79, 7413–7418.
Hata, A., Furumai, H., & Katayama, H. (2020). Sequential treatment using a hydrophobic resin and gel filtration to improve viral gene quantification from highly complex environmental concentrates. Water Research, 174, 115652.
Hata, A., Inaba, M., Katayama, H., & Furumai, H. (2017). Characterization of natural organic substances potentially hindering RT-PCR-based virus detection in large volumes of environmental water. Environmental Science and Technology, 51, 13568–13579.
Hata, A., Katayama, H., & Furumai, H. (2015). Organic substances interfere with reverse transcription-quantitative PCR-based virus detection in water samples. Applied and Environmental Microbiology, 81, 1585–1593.
Hata, A., Katayama, H., Kitajima, M., Visvanathan, C., Nol, C., & Furumai, H. (2011). Validation of internal controls for extraction and amplification of nucleic acids from enteric viruses in water samples. Applied and Environmental Microbiology, 77, 4336–4343.
Hennechart-Collette, C., Martin-Latil, S., Guillier, L., & Perelle, S. (2015). Determination of which virus to use as a process control when testing for the presence of hepatitis A virus and norovirus in food and water. International Journal of Food Microbiology, 202, 57–65.
Hewitt, J., Leonard, M., Greening, G. E., & Lewis, G. D. (2011). Influence of wastewater treatment process and the population size on human virus profiles in wastewater. Water Research, 45, 6267–6276.
Hill, V. R., Kahler, A. M., Jothikumar, N., Johnson, T. B., Hahn, D., & Cromeans, T. L. (2007). Multistate evaluation of an ultrafiltration-based procedure for simultaneous recovery of enteric microbes in 100-liter tap water samples. Applied and Environmental Microbiology, 73, 4218–4225.
Hill, V. R., Polaczyk, A. L., Hahn, D., Narayanan, J., Cromeans, T. L., Roberts, J. M., & Amburgey, J. E. (2005). Development of a rapid method for simultaneous recovery of diverse microbes in drinking water by ultrafiltration with sodium polyphosphate and surfactants. Applied and Environmental Microbiology, 71, 6878–6884.
Holowecky, P. M., James, R. R., Lorch, D. P., Straka, S. E., & Lindquist, H. D. A. (2009). Evaluation of ultrafiltration cartridges for a water sampling apparatus. Journal of Applied Microbiology, 106, 738–747.
Ikner, L. A., Gerba, C. P., & Bright, K. R. (2012). Concentration and recovery of viruses from water: A comprehensive review. Food and Environmental Virology, 4, 41–67.
Langenfeld, K., Chin, K., Roy, A., Wigginton, K., & Duhaime, M. B. (2021). Comparison of ultrafiltration and iron chloride flocculation in the preparation of aquatic viromes from contrasting sample types. PeerJ, 9, 1–32.
Liu, M., Hata, A., Katayama, H., & Kasuga, I. (2020). Consecutive ultrafiltration and silica adsorption for recovery of extracellular antibiotic resistance genes from an urban river. Environmental Pollution, 260, 114062.
Lytle, C. D., & Routson, L. B. (1995). Minimized virus binding for tests of barrier materials. Applied and Environmental Microbiology, 61, 643–649.
Kageyama, T., Kojima, S., Shinohara, M., Uchida, K., Fukushi, S., Hoshino, F. B., Takeda, N., & Katayama, K. (2003). Broadly reactive and highly sensitive assay for Norwalk-like viruses based on real-time quantitative reverse transcription-PCR. Journal of Clinical Microbiology, 41, 1548–1557.
Kahler, A., Johnson, T., Hahn, D., Narayanan, J., Derado, G., & Hill, V. (2015). Evaluation of an ultrafiltration-based procedure for simultaneous recovery of diverse microbes in source waters. Water, 7, 1202–1216.
Kermekchiev, M. B., Kirilova, L. I., Vail, E. E., & Barnes, W. M. (2009). Mutants of Taq DNA polymerase resistant to PCR inhibitors allow DNA amplification from whole blood and crude soil samples. Nucleic Acids Research, 37, e40.
Kitajima, M., Hata, A., Yamashita, T., Haramoto, E., Minagawa, H., & Katayama, H. (2013). Development of a reverse transcription-quantitative PCR system for detection and genotyping of Aichi viruses in clinical and environmental samples. Applied and Environmental Microbiology, 79, 3952–3958.
Kitajima, M., Sassi, H. P., & Torrey, J. R. (2018). Pepper mild mottle virus as a water quality indicator. NPJ Clean Water, 1, 19.
Kitajima, M., Tohya, Y., Matsubara, K., Haramoto, E., Utagawa, E., Katayama, H., & Ohgaki, S. (2008). Use of murine norovirus as a novel surrogate to evaluate resistance of human norovirus to free chlorine disinfection in drinking water supply system. Environmental Engineering Research, 45, 361–370. in Japanese.
Kreader, C. A. (1996). Relief of amplification inhibition in PCR with bovine serum albumin or T4 gene 32 protein. Applied and Environmental Microbiology, 62, 1102–1106.
Lewis, G. D., & Metcalf, T. G. (1988). Polyethylene glycol precipitation for recovery of pathogenic viruses, including hepatitis A virus and human rotavirus, from oyster, water, and sediment samples. Applied and Environmental Microbiology, 54, 1983–1988.
Masclaux, F. G., Hotz, P., Friedli, D., Savova-Bianchi, D., & Oppliger, A. (2013). High occurrence of hepatitis E virus in samples from wastewater treatment plants in Switzerland and comparison with other enteric viruses. Water Research, 47, 5101–5109.
McMinn, B. R., Huff, E. M., Rhodes, E. R., & Korajkic, A. (2017). Concentration and quantification of somatic and F+ coliphages from recreational waters. Journal of Virological Methods, 249, 58–65.
Miura, T., Masago, Y., Sano, D., & Omura, T. (2011). Development of an effective method of viral genomic RNA recovery from environmental silty sediments for quantitative molecular detection. Applied and Environmental Microbiology, 77, 3975–3981.
Mooijman, K. A., Bahar, M., Muniesa, M., & Havelaar, A. H. (2002). Optimisation of ISO 10705–1 on enumeration of F-specific bacteriophages. Journal of Virological Methods, 103, 129–136.
Mueller, J. A., Culley, A. I., & Steward, G. F. (2014). Variables influencing extraction of nucleic acids from microbial plankton (viruses, bacteria, and protists) collected on nanoporous aluminum oxide filters. Applied and Environmental Microbiology, 80, 3930–3942.
Mull, B., & Hill, V. R. (2012). Recovery of diverse microbes in high turbidity surface water samples using dead-end ultra filtration. Journal of Microbiological Methods, 91, 429–433.
Murphy, J. L., Kahler, A. M., Nansubug, I., Nanyunj, E. M., Kaplan, B., Jothikumar, N., Routh, J., Gómez, G. A., Mintz, E. D., & Hill, V. R. (2017). Environmental survey of drinking water sources in Kampala, Uganda, during a typhoid fever outbreak. Applied and Environmental Microbiology, 83, e01706-e1717.
Rajal, V. B., McSwain, B. S., Thompson, D. E., Leutenegger, C. M., Kildare, B. J., & Wuertz, S. (2007). Validation of hollow fiber ultrafiltration and real-time PCR using bacteriophage PP7 as surrogate for the quantification of viruses from water samples. Water Research, 41, 1411–1422.
R Core Team. (2020). R: A language and environment for statistical computing. R Foundation for Statistical Computing.
Rhodes, E. R., Huff, E. M., Hamilton, D. W., & Jones, J. L. (2016). The evaluation of hollow-fiber ultrafiltration and celite concentration of enteroviruses, adenoviruses and bacteriophage from different water matrices. Journal of Virological Methods, 228, 31–38.
Rodríguez, R. A., Thie, L., Gibbons, C. D., & Sobsey, M. D. (2012). Reducing the effects of environmental inhibition in quantitative real-time PCR detection of adenovirus and norovirus in recreational seawaters. Journal of Virological Methods, 181, 43–50.
Rosiles-González, G., Ávila-Torres, G., Moreno-Valenzuela, O. A., Cháidez-Quiroz, C., Hernández-Flores, C. I., Acosta-González, G., Brown, J. K., Betancourt, W. Q., Gerba, C. P., & Hernández-Zepeda, C. (2019). Norovirus and human adenovirus occurrence and diversity in recreational water in a karst aquifer in the Yucatan Peninsula, Mexico. Journal of Applied Microbiology, 127, 1255–1269.
Schrader, C., Schielke, A., Ellerbroek, L., & Johne, R. (2012). PCR inhibitors—Occurrence, properties and removal. Journal of Applied Microbiology, 113, 1014–1026.
Sidstedt, M., Rådström, P., & Hedman, J. (2020). PCR inhibition in qPCR, dPCR and MPS—Mechanisms and solutions. Analytical and Bioanalytical Chemistry, 412, 2009–2023.
Sincero, T. C. M., Levin, D. B., Simões, C. M. O., & Barardi, C. R. M. (2006). Detection of hepatitis A virus (HAV) in oysters (Crassostrea gigas). Water Research, 40, 895–902.
Smith, C. M., & Hill, V. R. (2009). Dead-end hollow-fiber ultrafiltration for recovery of diverse microbes from water. Applied and Environmental Microbiology, 75, 5284–5289.
Symonds, E. M., Nguyen, K. H., Harwood, V. J., & Breitbart, M. (2018). Pepper mild mottle virus: A plant pathogen with a greater purpose in (waste)water treatment development and public health management. Water Research, 144, 1–12.
Torii, S., Furumai, H., & Katayama, H. (2021). Applicability of polyethylene glycol precipitation followed by acid guanidinium thiocyanate–phenol–chloroform extraction for the detection of SARS-CoV-2 RNA from municipal wastewater. Science of the Total Environment, 756, 143067.
Uhrbrand, K., Myrmel, M., Maunula, L., Vainio, K., Trebbien, R., Nørrung, B., & Schultz, A. C. (2010). Evaluation of a rapid method for recovery of norovirus and hepatitis A virus from oysters and blue mussels. Journal of Virological Methods, 169, 70–78.
Varughese, E. A., Brinkman, N. E., Anneken, E. M., Cashdollar, J. L., Fout, G. S., Furlong, E. T., Kolpin, D. W., Glassmeyer, S. T., & Keely, S. P. (2018). Estimating virus occurrence using Bayesian modeling in multiple drinking water systems of the United States. Science of the Total Environment, 619–620, 1330–1339.
Wobus, C. E., Karst, S. M., Thackray, L. B., Chang, K.-O., Sosnovtsev, S. V., Belliot, G., Krug, A., Mackenzie, J. M., Green, K. Y., & Virgin, H. W. (2004). Replication of Norovirus in cell culture reveals a tropism for dendritic cells and macrophages. PLoS Biology, 2, e432.
Wolf, S., Hewitt, J., & Greening, G. E. (2010). Viral multiplex quantitative PCR assays for tracking sources of fecal contamination. Applied and Environmental Microbiology, 76, 1388–1394.
World Health Organization, WHO. (2017). Guidelines for drinking-water quality (4th ed.). WHO.
Worley-Morse, T., Mann, M., Khunjar, W., Olabode, L., & Gonzalez, R. (2019). Evaluating the fate of bacterial indicators, viral indicators, and viruses in water resource recovery facilities. Water Environment Research, 91, 830–842.
Acknowledgements
This work was supported by JSPS KAKENHI (Grant Numbers 17H03332, 18K13857, 20H02284, and 20H00259) from the Japan Society for the Promotion of Science and The Tokyu Foundation, Japan (Grant Number 2019-112).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial or non-financial interests that are directly or indirectly related to the work reported in this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hata, A., Meuchi, Y., Liu, M. et al. Surfactant Treatment for Efficient Gene Detection of Enteric Viruses and Indicators in Surface Water Concentrated by Ultrafiltration. Food Environ Virol 15, 8–20 (2023). https://doi.org/10.1007/s12560-022-09543-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12560-022-09543-y