Abstract
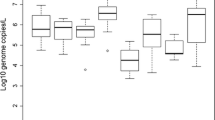
This study was conducted to evaluate the microbiological quality of a mangrove estuary in the Vitória Bay region, Espírito Santo, Brazil. We analyzed the presence and concentration of enteric viruses and thermotolerant coliforms in water, mussels (Mytella charruana and Mytella guyanensis), and oysters (Crassostrea rhizophorae), collected over a 13-month period. Human adenovirus, rotavirus A (RVA), and norovirus genogroup II were analyzed by quantitative PCR. The highest viral load was found in RVA-positive samples with a concentration of 3.0 × 104 genome copies (GC) L−1 in water samples and 1.3 × 105 GC g−1 in bivalves. RVA was the most prevalent virus in all matrices. Thermotolerant coliforms were quantified as colony-forming units (CFU) by the membrane filtration method. The concentration of these bacteria in water was in accordance with the Brazilian standard for recreational waters (< 250 CFU 100 mL−1) during most of the monitoring period (12 out of 13 months). However, thermotolerant coliform concentrations of 3.0, 3.1, and 2.6 log CFU 100 g−1 were detected in M. charruana, M. guyanensis, and C. rhizophorae, respectively. The presence of human-specific viruses in water and bivalves reflects the strong anthropogenic impact on the mangrove and serves as an early warning of waterborne and foodborne disease outbreaks resulting from the consumption of shellfish and the practice of water recreational activities in the region.




Similar content being viewed by others
References
Alfano-Sobsey, E., Sweat, D., Hall, A., Breedlove, F., Rodriguez, R., Greene, S., et al. (2012). Norovirus outbreak associated with undercooked oysters and secondary household transmission. Epidemiology and Infection, 140(2), 276–282.
Allard, A., Albinsson, B., & Wadell, G. (2001). Rapid typing of human adenoviruses by a general PCR combined with restriction endonuclease analysis. Journal of Clinical Microbiology, 39(12), 498–505.
Amaral, M. S. C., Estevam, G. K., Penatti, M., Lafontaine, R., Lima, I. C. G., Spada, P. K. P., et al. (2015). The prevalence of norovirus, astrovirus and adenovirus infections among hospitalised children with acute gastroenteritis in Porto Velho, state of Rondônia, western Brazilian Amazon. Memórias do Instituto Oswaldo Cruz, 110, 215–221.
APHA; AWWA; WEF. (2005). Standard methods for examination of water and wastewater. American Public Health Association, New York.
Barbirato, J. O., Conceição, J. M., Cabral, D. S., Barroso, A. L. P., Zandonadi, D. B., & Dobbss, L. B. (2016). Microbiological monitoring in two areas with different levels of conservation in the mangroves of an Ecological Station, Vitória, ES. Acta Scientiarum Biological Sciences Maringá, 38(3), 333–339.
Bellou, M., Kokkino, S. P., & Vantarakis, A. (2013). Shellfish-borne viral outbreaks: A systematic review. Food and Environmental Virology, 5(1), 13–23.
Boom, R., Sol, C. J., Salimans, M. M., Jansen, C. L., Wertheim-Van, D. P. M., & Van Der Noordaa, J. (1990). Rapid and simple method for purification of nucleic acids. Journal of Clinical Microbiology, 28, 495–503.
Brazil. (2000). Ministério do Meio Ambiente. Conselho Nacional de Meio Ambiente (CONAMA). Resolução 274 de 29 de Novembro de 2000.
Brazil. (2005). Ministério do Meio Ambiente. Conselho Nacional de Meio Ambiente (CONAMA). Resolução nº 357 de 17 de Março de 2005.
Campos, C. J. A., & Lees, D. N. (2014). Environmental transmission of human noroviruses in shellfish waters. Applied and Environmental Microbiology, 80(12), 3552–3561.
Dore, B., Keaveneyl, S., Flannery, J., & Rajko-Nenow, P. (2010). Management of health risks associated with oysters harvested from a norovirus contaminated area, Ireland. Eurosurveillance, 15(19), 1–4.
Ferreira, F. F., Guimarães, F. R., Fumian, T. M., Victoria, M., Vieira, C. B., Luz, S., et al. (2009). Environmental dissemination of group A rotavirus: P-type, G-type and subgroup characterization. Water Science and Technology, 60(3), 633–642.
Formiga-Cruz, M., Tofiño-Quesada, G., Bofill-Mas, S., Lees, D. N., Henshilwood, K., Allard, A. K., et al. (2002). Distribution of human virus contamination in shellfish from different growing areas in Greece, Spain, Sweden, and the United Kingdom. Applied and Environmental Microbiology, 68(12), 5990–5998.
Girones, R., Puig, M., Allard, A., Lucena, F., Wadell, G., & Jofre, J. (1995). Detection of adenovirus and enterovirus by PCR amplification in polluted waters. Water Science and Technology, 31(5–6), 351–357.
Guillois-Bécel, E., Couturier, J. C., Le Saux, A. M., Roque-Afonso, F. S., Le Guyader, A., Le Goas, J., et al. (2009). An oyster-associated hepatitis A outbreak in France in 2007. Euro Surveillance, 14(10), 19144.
Hafliger, D., Gilgen, M., Luthy, J., & Hubner, P. (1997). Seminested RT-PCR systems for small round structured viruses and detection of enteric viruses seafood. International Journal of Food Microbiology, 37(1), 27–36.
Hassard, F., Sharp, J. H., Taft, H., Le Vay, L., Harris, J. P., McDonald, J. E., et al. (2017). Critical review on the public health impact of norovirus contamination in shellfish and the environment: A UK perspective. Food Environmental Virology, 9, 123–141.
Hernroth, B. E., Conden-Hansson, A. C., Rehnstam-Holm, A. S., Girones, R., & Allard, A. K. (2002). Environmental factors influencing human viral pathogens and their potential indicator organisms in the blue mussel, Mytilus edulis: The first Scandinavian report. Applied Environmental Microbiology, 68(9), 4523–4533.
International Standart (ISO 16140). (2003). Microbiology of food and animal feeding stuffs–Protocol for the validation of alternative methods.
Iturriza-Gomara, M., Green, J., Brown, D. W. G., Desslberger, U., & Gray, J. J. (1999). Comparison of specific and random priming in the reverse transcriptase polymerase chain reaction for genotyping group A rotaviruses. Journal of Virological Methods, 78(1–2), 93–103.
Jesus, H. C., Costa, E. A., Mendonça, A. S. F., & Zandonade, E. (2004). Distribuição de metais pesados em sedimentos do sistema estuarino da ilha de Vitória-ES. Química Nova, 27(3), 378–386.
Kageyama, T., Kojima, S., Shinohara, M., Uchida, K., Fukushi, S., & Hoshino, F. B. (2003). Broadly reactive and highly sensitive assay for norwalk-like viruses based on real-time quantitative reverse transcription-PCR. Journal of Clinical Microbiology, 41(4), 1548–1557.
Katayama, H., Shimasaki, A., & Ohgaki, S. (2002). Development of a virus concentration method and its application to detection of enterovirus and Norwalk virus from coastal seawater. Applied and Environmental Microbiology, 68(3), 1033–1039.
Kathiresan, K., & Bingham, B. L. (2001). Biology of mangroves and mangrove ecosystems. Advances in Marine Biology, 40, 81–251.
Kauppinen, A., Ilkka, T., & Miettinen, I. T. (2017). Persistence of norovirus GII genome in drinking water and wastewater at different temperatures. Pathogens, 6, 48. https://doi.org/10.3390/pathogens6040048.
Keller, R., Justino, J. F., & Cassini, S. T. (2013). Assessment of water and seafood microbiology quality in a mangrove region in Vitória, Brazil. Journal of Water and Health, 11(3), 573–580.
Lanata, C. F., Fischer-Walker, C. L., Olascoaga, A. C., Torres, C. X., Aryee, M. J., & Black, R. E. (2013). Global causes of diarrheal disease mortality in children < 5 years of age: A systematic review. PLoS ONE, 8(9), e72788.
Le Guyader, F., Haugarreau, L., Miossec, L., Dubois, E., & Pommepuy, M. (2000). Three-year study to assess human enteric viruses in shellfish. Applied and Environmental Microbiology, 66(8), 3241–3248.
Le Guyader, F., Le Saux, J. C., Ambert-Balay, K., Krol, J., Serais, O., Parnaudeau, S., et al. (2008). Aichi virus, norovirus, astrovirus, enterovirus, and rotavirus involved in clinical cases from a French oyster-related gastroenteritis outbreak. Journal of Clinical Microbiology, 46(12), 4011–4017.
Le Guyader, F. S., Parnaudeau, S., Schaeffer, J., Bosch, A., Loisy, F., Pommepuy, M., et al. (2009). Detection and quantification of noroviruses in shellfish. Applied and Environmental Microbiology, 75(3), 618–624.
Le Mennec, C., Parnaudeau, S., Rumebe, M., Le Saux, J., Piquet, J., & Le Guyader, S. F. (2017). Follow-up of norovirus contamination in an oyster production area linked to repeated outbreaks. Food and Environmental Virology, 9(1), 54–61.
Lees, D. (2000). Viruses and bivalve shellfish. International Journal of Food Microbiology, 59, 81–116.
Lowther, J. A., Gustar, N. E., Powell, A. L., Hartnell, R. E., & Lees, D. N. (2012). Two-year systematic study to assess norovirus contamination in oysters from commercial harvesting areas in the United Kingdom. Applied and Environmental Microbiology, 78(16), 5812–5817.
Marino, A., Lombardo, L., & Fiorentino, C. (2005). Uptake of Escherichia coli, Vibrio cholerae and Enterococcus durans by, and depuration of mussels (Mytilus galloprovincialis). International Journal Food Microbiology, 99(3), 281–286.
Miagostovich, M. P., Ferreira, F. F. M., Guimarães, F. R., Fumian, T. M., Diniz-Mendes, L., Luz, S. L. B., et al. (2008). Molecular detection and characterization of gastroenteritis viruses occurring naturally in the stream waters of Manaus, Central Amazônia, Brazil. Applied and Environmental Microbiology, 74(2), 375–382.
Morse, D. L., Guzewich, J. J., Hanrahan, J. P., Stricof, R., Shayegani, M., Deibel, R., et al. (1986). Widespread outbreaks of clam- and oyster-associated gastroenteritis. The New England Journal of Medicine, 314, 678–681.
Mounts, A. W., Ando, T., Koopmans, M., Bresee, J. S., Noel, J., et al. (2000). Cold weather seasonality of gastroenteritis associated with Norwalk-like viruses. The Journal of Infectious Diseases, 181(2), 284–287.
Oliveira, J., Cunha, A., Castilho, F., Romalde, J. L., & Pereira, M. J. (2011). Microbial contamination and purification of bivalve shellfish: Crucial aspects in monitoring and future perspectives—A mini-review. Food Control, 22(6), 805–816.
Osuolale, O., & Okoh, A. (2015). Incidence of human adenoviruses and Hepatitis A virus in the final effluent of selected wastewater treatment plants in Eastern Cape Province, South Africa. Virology Journal, 12(98), 1–8.
Parashar, U. D., Gibson, C. J., Bresee, J. S., & Glass, R. I. (2006). Rotavirus and severe childhood diarrhea. Emerging Infectious Diseases, 12(2), 304–306.
Phanuwan, C., Takizawa, S., Oguma, K., Katayama, H., Yunika, A., & Ohgaki, S. (2006). Monitoring of human enteric viruses and coliform bacteria in waters after urban flood in Jakarta, Indonesia. Water Science & Technology, 54(3), 203–210.
Pina, S., Montserrat, P., Lucena, F., Jofre, J., & Girones, R. (1998). Viral pollution in the environment and in shellfish: Human adenovirus detection by PCR as an index of human viruses. Applied and Environmental Microbiology, 64(9), 3376–3382.
Portes, S. A. R., Volotão, E. M., Rocha, M. S., Rebelo, M. C., Xavier, M. P. T. P., Assis, R. M., et al. (2016). A non-enteric adenovirus A12 gastroenteritis outbreak in Rio de Janeiro, Brazil. Memórias do Instituto Oswaldo Cruz, 111(6), 403–406.
Potasman, I., Paz, A., & Odeh, M. (2002). Infectious outbreaks associated with bivalve shellfish consumption: A worldwide perspective. Clinical Infectious Diseases, 35(8), 921–928.
Prato, R., Lopalco, P. L., Chironna, M., Barbuti, G., Germinario, C., & Quarto, M. (2004). Norovirus gastroenteritis general outbreak associated with raw shellfish consumption in South Italy. BMC Infectious Diseases, 4, 37.
Rajko-Nenow, P., Keaveney, S., Flannery, J., O’Flaherty, V., & Doré, W. (2012). Characterization of norovirus contamination in an Irish shellfishery using real-time RT-qPCR and sequencing analysis. International Journal of Food Microbiology, 160(2), 105–112.
Rezaei, M., Sohrabi, A., Edalat, R., Siadat, S. D., Gomari, H., Rezaei, M., et al. (2012). Molecular epidemiology of acute gastroenteritis caused by subgenus F (40, 41) enteric adenoviruses in inpatient children. Labmedicine, 43(1), 10–15.
Romalde, J. L., Area, E., Sánchez, G., Ribao, C., Torrado, I., Abad, X., et al. (2002). Prevalence of enterovirus and hepatitis A virus in molluscs from Galicia (NW Spain). Inadequacy of the EU standards of microbiological quality. International Journal of Food Microbiology, 74(12), 119–130.
Schlindwein, A. D., Rigotto, C., Simoes, C. M. O., & Barardi, C. R. M. (2010). Detection of enteric viruses in sewage sludge and treated wastewater effluent. Water Science Technology, 61(2), 537–544.
Seitz, S. R., Leon, J. S., Schwab, K. J., Lyon, G. M., Dowd, M., McDaniels, M., et al. (2011). Norovirus infectivity in humans and persistence in water. Applied and Environmental Microbiology, 77(19), 6884–6888.
Smith, A. J., McCarthy, N., Saldana, L., Ihekweazu, C., McPhedran, K., Adak, G. K., et al. (2012). A large foodborne outbreak of norovirus in diners at a restaurant in England between January and February 2009. Epidemiology and Infection, 140(9), 1695–1701.
Souza, D. S. M., Ramos, A. P. D., Nunes, F. F., Moresco, V., Taniguchi, S., Leal, D. A. G., et al. (2012). Evaluation of tropical water sources and mollusks in southern Brazil using microbiological, biochemical, and chemical parameters. Ecotoxicology and Environmental Safety, 76(2), 153–161.
Teunis, P. F., Moe, C. L., Liu, P., Miller, S. E., Lindesmith, L., & Baric, R. S. (2008). Norwalk virus: How infectious is it? Journal of Medical Virology, 80(8), 1468–1476.
Traore, O., Arnal, C., Mignotte, B., Maul, A., Laveran, H., Billaudel, S., et al. (1998). Reverse transcriptase PCR detection of astrovirus, hepatitis A virus, and poliovirus in experimentally contaminated mussels: Comparison of several extraction and concentration methods. Applied and Environmental Microbiology, 64, 3118–3122.
Uhnoo, I., Wadell, G., Svensson, L., & Johansson, M. E. (1984). Importance of enteric adenoviruses 40 and 41 in acute gastroenteritis in infants and young children. Journal of Clinical Microbiology, 20(3), 365–372.
Victoria, M., Fumian, T. M., Rocha, M. S., Dalmao, F., Leite, J. P. G., Girones, R., et al. (2014). Gastroenteric virus dissemination and influence of rainfall events in urban beaches in Brazil. Journal of Applied Microbiology, 117, 1210–1218.
Vilariño, M. L., Le Guyader, F. S., Polo, D., Schaeffer, J., Kröl, J., & Romalde, J. L. (2009). Assessment of human enteric viruses in cultured and wild bivalve molluscs. International Microbiology, 12, 145–151.
Wall, R., Dymond, N., Bell, A., Thornley, C., Buik, H., Cumming, D., et al. (2011). Two New Zealand outbreaks of norovirus gastroenteritis linked to commercially farmed oysters. New Zealand Medicine Journal, 124(1347), 63–71.
Winkler, L. W. (1888). Die Bestimmung des in Wasser gelösten Sauerstoffen. Berichte der Deutschen Chemischen Gesellschaft, 21, 2843–2855.
Zamprogno, G. C., Tognella, M. M. P., Quaresma, V. S., Costa, M. B., Pascoalini, S. S., & Couto, G. F. (2016). The structural heterogeneity of an urbanised mangrove forest area in southeastern Brazil: Influence of environmental factors and anthropogenic stressors. Brazilian Journal of Oceanography, 64(2), 157–172.
Zeng, S. Q., Halkosaloa, A., Salminena, M., Szakala, E. D., Puustinena, L., & Vesikaria, T. (2008). One-step quantitative RT-PCR for the detection of rotavirus in acute gastroenteritis. Journal of Virological Methods, 153(2), 238–240.
Acknowledgments
This work was supported by the Brazilian National Research Council (CNPq; Grant No. 14/2009). We thank the Brazilian Federal Agency for Support and Evaluation of Graduate Education (CAPES) for providing a scholarship to Rodrigo Pratte-Santos, Karolina Scarpati, Sara Angelino Martins, and Suzanne Mariane Loss. We also thank Dr. Juliana Justino of the Laboratory of Applied Genetics for Biodiversity Conservation for providing laboratory assistance and conducting the DNA sequencing analysis.
Funding
Funding was provided by Brazilian National Research Council (CNPq, Grant No. 14/2009).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
12560_2019_9391_MOESM1_ESM.tif
Fig. S1 Temporal distribution of thermotolerant coliforms in water from January to December 2011 and in mussels (Mytella charruana and Mytella guyanensis) and oysters (Crassostrea rhizophorae) from January 2011 to January 2012 (TIFF 228 kb)
Rights and permissions
About this article
Cite this article
Keller, R., Pratte-Santos, R., Scarpati, K. et al. Surveillance of Enteric Viruses and Thermotolerant Coliforms in Surface Water and Bivalves from a Mangrove Estuary in Southeastern Brazil. Food Environ Virol 11, 288–296 (2019). https://doi.org/10.1007/s12560-019-09391-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12560-019-09391-3