Abstract
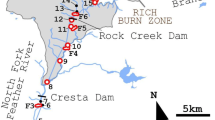
This paper reports a study of norovirus (NoV) GII distribution and persistence in Sydney rock oysters (SRO) (Saccostrea glomerata) located in an estuary after a pump station sewage overflow. SRO were strategically placed at six sites spanning the length of the estuary from the pump station to the sea. The spatial and temporal distribution of NoV, hepatitis A virus (HAV) and Escherichia coli (E. coli) in oysters was mapped after the contamination event. NoV GI and GII, HAV and E. coli were quantified for up to 48 days in oysters placed at six sites ranging from 0.05 to 8.20 km from the sewage overflow. NoV GII was detected up to 5.29 km downstream and persisted in oysters for 42 days at the site closest to the overflow. NoV GII concentrations decreased significantly over time; a reduction rate of 8.5% per day was observed in oysters (p < 0.001). NoV GII concentrations decreased significantly as a function of distance at a rate of 5.8% per km (p < 0.001) and the decline in E. coli concentration with distance was 20.1% per km (p < 0.001). HAV and NoV GI were not detected. A comparison of NoV GII reduction rates from oysters over time, as observed in this study and other published research, collectively suggest that GII reduction rates from oysters may be broadly similar, regardless of environmental conditions, oyster species and genotype.




Similar content being viewed by others
References
Anonymous. (2001–2011). OzFoodNet quarterly reports. Communication Disease Intelligence Quarterly Report, 25–35.
Anonymous. (2005). ISO/TS 16649-3:2005 Microbiology of food and animal feeding stuffs–Horizontal method for the enumeration of beta-glucuronidase-positive Escherichia coli–Part 3: Most probable number technique using 5-bromo-4-chloro-3-indolyl-beta-d-glucuronide. Microbiology of food and animal feeding stuffs. Geneva: International Organization of Standardization.
Anonymous. (2013). ISO/TS 15216-1:2013 Microbiology of food and animal feed–Horizontal method for determination of hepatitis A virus and norovirus in food using real-time RT-PCR–Part 1: Method for quantification. Geneva: International Organization of Standardization
Bougeard, M., Le Saux, J. C., Pérenne, N., Baffaut, C., Robin, M., & Pommepuy, M. (2011). Modeling of Escherichia coli fluxes on a catchment and the impact on coastal water and shellfish quality. Journal of American Water Resources Association, 47(2), 350–366.
Brake, F. A., Ross, T., Kiermeier, A., Holds, G., & McLeod, C. (2014). A survey of Australian oysters for the presence of human noroviruses. Food Microbiology, 44, 264–270.
Bureau of Meteorology Daily rainfall Mullumbimby (1954 - current-b). Australian Government. Retrieved November, 2015, fromhttp://www.bom.gov.au/climate/data.
Burkhardt, W., III, Calci, K. R., Watkins, W. D., Rippey, S. R., & Chirtel, S. J. (2000). Inactivation of indicator microorganisms in estuarine waters. Water Research, 34(8), 2207–2214. doi:10.1016/s0043-1354(99)00399-1.
Campos, C. J. A., Kershaw, S., Lee, R. J., Morgan, O. C., & Hargin, K. (2011). Rainfall and river flows are predictors for beta-glucuronidase positive Escherichia coli accumulation in mussels and Pacific oysters from the Dart Estuary (England). Journal Water Health, 9(2), 368–381.
Crowther, J., Kay, D., & Wyer, M. D. (2001). Relationships between microbial water quality and environmental conditions in coastal recreational waters: The Fylde Coast, UK. Water Research, 35(17), 4029–4038.
da Silva, A. K., Le Saux, J.-C., Parnaudeau, S., Pommepuy, M., Elimelech, M., & Le Guyader, F. S. (2007). Evaluation of removal of noroviruses during wastewater treatment, using real-time reverse transcription-PCR: Different behaviors of genogroups I and II. Applied and Environmental Microbiology, 73(24), 7891–7897. doi:10.1128/aem.01428-07.
Department of Primary Industry Water Freshwater discharge, Durrumbul Gauge, NSW (1954–2014). Department of Primary Industry - Water, NSW, Australia. Retrieved January, 2014, from http://waterinfo.nsw.gov.au/pinneena/cm.shtml.
Donovan, T. J., Gallacher, S., Andrews, N. J., Greenwood, M. H., Graham, J., Russell, J. E., et al. (1998). Modification of the standard method used in the United Kingdom for counting Escherichia coli in live bivalve molluscs. Communicable Diseases & Public Health/P.H.L.S., 1(3), 188–196.
Dore, B., Keaveney, S., Flannery, J., & Rajko-Nenow, P. (2010). Management of health risks associated with oysters harvested from a norovirus contaminated area, Ireland, February–March 2010. European Surveillance, 15(19), 19567.
Ferguson, A., Eyre, B., & Gay, J. (2004). Nutrient cycling in the sub-tropical Brunswick estuary, Australia. Estuaries, 27(1), 1–17.
Goblick, G. N., Anbarchian, J. M., Woods, J., Burkhardt, W., III, & Calci, K. (2011). Evaluating the dilution of wastewater treatment plant effluent and viral impacts on shellfish growing areas in Mobile Bay, Alabama. Journal of Shellfish Research, 30(3), 979–987.
Greening, G. E., & Hewitt, J. (2008). Norovirus detection in shellfish using a rapid, sensitive virus recovery and real-time RT-PCR detection protocol. Food Analytical Methods, 1(2), 109–118. doi:10.1007/s12161-008-9018-3.
Greening, G. E., Hewitt, J., Hay, B. E., & Grant, C. M. (2003). Persistence of Norwalk-like viruses over time in Pacific oysters grown in the natural environment. In A. V. García (Ed.), 4th International Conference on Molluscan Shellfish Safety, Santiago de Compostela, Spain, 2003 (Vol. Molluscan Shellfish Safety: Proceedings of the 4th International Conference on Molluscan Shellfish Safety, Santiago de Compostela, Spain, June 4–8, 2002): ICMSS
Greening, G. E., & Lewis, G. D. (2007). Safeguarding environmental health and market access for NZ foods objective 2: Virus prevalence in shellfish (p. 45). Porirua, NZ: Institute of Environmental Science and Research.
Grodzki, M., Ollivier, J., Le Saux, J. C., Piquet, J. C., Noyer, M., & Le Guyader, F. S. (2012). Impact of Xynthia tempest on viral contamination of shellfish. Applied and Environmental Microbiology, 78(9), 3508–3511.
Hall, A. J., Eisenbart, V. G., Etingue, A. L., Gould, L. H., Lopman, B. A., & Parashar, U. D. (2012). Epidemiology of foodborne norovirus outbreaks, United States, 2001–2008. Emerging Infectious Diseases, 18(10), 1566–1573. doi:10.3201/eid1810.120833.
Kageyama, T., Kojima, S., Shinohara, M., Uchida, K., Fukushi, S., Hoshino, F. B., et al. (2003). Broadly reactive and highly sensitive assay for Norwalk-like viruses based on real-time quantitative reverse transcription-PCR. Journal of Clinical Microbiology, 41(4), 1548–1557.
Kingsley, D. H., & Richards, G. P. (2003). Persistence of HAV in oysters. Journal of Food Protection, 66, 331–334.
Le Guyader, F. S., Haugarreau, L., Miossec, L., Dubois, E., & Pommepuy, M. (2000). Three-year study to assess human enteric viruses in shellfish. Applied and Environmental Microbiology, 66(8), 3241–3248.
Lees, D. (2000). Viruses and bivalve shellfish. International Journal Food Microbiology, 59(1–2), 81–116. doi:10.1016/s0168-1605(00)00248-8.
Lowther, J. A., Gustar, N. E., Powell, A. L., Hartnell, R. E., & Lees, D. N. (2012). Two-year systematic study to assess norovirus contamination in oysters from commercial harvesting areas in the United Kingdom. Applied and Environmental Microbiology, 78(16), 5812–5817. doi:10.1128/aem.01046-12.
Maalouf, H., Schaeffer, J., Parnaudeau, S., Le Pendu, J., Atmar, R. L., Crawford, S. E., et al. (2011). Strain-dependent norovirus bioaccumulation in oysters. Applied and Environmental Microbiology, 77(10), 3189–3196. doi:10.1128/AEM.03010-10.
Manly Hydraulics Laboratory. (2005). Brunswick area sewerage augmentation project EIS report 1388. Sydney, NSW: NSW Department of Public Works and Services, Manly Hydraulics Laboratory.
Nappier, S. P., Graczyk, T. K., & Schwab, K. J. (2008). Bioaccumulation, retention, and depuration of enteric viruses by Crassostrea virginica and Crassostrea ariakensis oysters. Applied and Environmental Microbiology, 74(22), 6825–6831.
New Zealand Food Safety Authority. (2006). Animal products (specifications for Bivalve Molluscan Shellfish) (p. 77). Wellington, NZ: New Zealand Ministry for Primary Industries.
Patel, M. M., Hall, A. J., Vinjé, J., & Parashar, U. D. (2009). Noroviruses: A comprehensive review. Journal Clinical Virology, 44(1), 1–8.
R Core Team. (2013). R: A language and environment for statistical computing. (3.0.2 ed.). Vienna: R Foundation for Statistical Computing.
Rajko-Nenow, P., Waters, A., Keaveney, S., Flannery, J., Tuite, G., Coughlan, S., et al. (2013). Norovirus genotypes present in oysters and in effluent from a wastewater treatment plant during the seasonal peak of infections in ireland in 2010. Applied and Environmental Microbiology, 79(8), 2578–2587. doi:10.1186/1743-422X-8-310.
Richards, G. P., Watson, M. A., Fankhauser, R. L., & Monroe, S. S. (2004). Genogroup I and II noroviruses detected in stool samples by real-time reverse transcription-PCR using highly degenerate universal primers. Applied and Environmental Microbiology, 70(12), 7179–7184. doi:10.1128/aem.70.12.7179-7184.2004.
Scallan, E., Hoekstra, R. M., Angulo, F. J., Tauxe, R. V., Widdowson, M.-A., Roy, S. L., et al. (2011). Foodborne illness acquired in the United States—Major pathogens. Emerging Infectious Diseases, 17(1), 7–15.
Schwab, K. J., Neill, F. H., Estes, M. K., Metcalf, T. G., & Atmar, R. L. (1998). Distribution of Norwalk virus within shellfish following bioaccumulation and subsequent depuration by detection using RT-PCR. Journal Food Protection, 61(12), 1674–1680.
Seitz, S. R., Leon, J. S., Schwab, K. J., Lyon, G. M., Dowd, M., McDaniels, M., et al. (2011). Norovirus infectivity in humans and persistence in water. Applied and Environmental Microbiology, 77(19), 6884–6888. doi:10.1128/aem.05806-11.
U. S. Food and Drug Administration. (2015). National shellfish sanitation program (NSSP) guide for the control of molluscan shellfish. Revision 2015 (p. 464). Silver Springs, MD: U. S. Food and Drug Administration.
Walne, P. R. (1979). Culture of bivalve molluscs: 50 years’ experience at Conwy. Farnham, Surrey: Fishing News Books Ltd.
Acknowledgements
Oysters were collected from the estuary using a NSW Government Industry and Investment Scientific Collection Permit no. P09/001-2.0 as per section 37, Fisheries Management Act 1994. Laboratory analyses were conducted at South Australian Research & Development Institute, South Australia. Kate Hodgson is thanked for performing part of the HAV testing. NSW State Shellfish regulator Anthony Zammit identified the survey site. Geoffrey Lawler, an oyster farmer from NSW kindly helped with provision, installation and sampling of the oysters. Brenda Hay (AquaBio Consultants Ltd. NZ), Peter Rees, Manager Utilities, Infrastructure Services (Byron Shire Council, NSA), Dr. Rod Ratcliffe (SA Pathology) and David Allsop (Manly Hydraulics Laboratory NSW) are thanked for their advice. The authors gratefully acknowledge the funding from NSWFA. Felicity Brake was supported with funding from the Australian Seafood CRC, grant number 2008/741.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Brake, F., Kiermeier, A., Ross, T. et al. Spatial and Temporal Distribution of Norovirus and E. coli in Sydney Rock Oysters Following a Sewage Overflow into an Estuary. Food Environ Virol 10, 7–15 (2018). https://doi.org/10.1007/s12560-017-9313-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12560-017-9313-5