Abstract
The Cambrian Explosion by nature is a three-phased explosion of animal body plans alongside episodic biomineralization, pulsed change of generic diversity, body size variation, and progressive increase of ecosystem complexity. The Cambrian was a time of crown groups nested by numbers of stem groups with a high-rank taxonomy of Linnaean system (classes and above). Some stem groups temporarily succeeded while others were ephemeral and underrepresented by few taxa. The high number of stem groups in the early history of animals is a major reason for morphological gaps across phyla that we see today. Most phylum-level clades achieved their maximal disparity (or morphological breadth) during the time interval close to their first appearance in the fossil record during the early Cambrian, whereas others, principally arthropods and chordates, exhibit a progressive exploration of morphospace in subsequent Phanerozoic. The overall envelope of metazoan morphospace occupation was already broad in the early Cambrian though it did not reach maximal disparity nor has diminished significantly as a consequence of extinction since the Cambrian. Intrinsic and extrinsic causes were extensively discussed but they are merely prerequisites for the Cambrian Explosion. Without the molecular evolution, there could be no Cambrian Explosion. However, the developmental system is alone insufficient to explain Cambrian Explosion. Time-equivalent environmental changes were often considered as extrinsic causes, but the time coincidence is also insufficient to establish causality. Like any other evolutionary event, it is the ecology that make the Cambrian Explosion possible though ecological processes failed to cause a burst of new body plans in the subsequent evolutionary radiations. The Cambrian Explosion is a polythetic event in natural history and manifested in many aspects. No simple, single cause can explain the entire phenomenon.
Similar content being viewed by others
Introduction
The Cambrian Explosion is by far the most dramatic chapter in the annals of animal evolutionary history, which laid foundations for animal evolution since the Phanerozoic. The topic has been frequently reviewed by multiple authors and in multiple languages (e.g., Seilacher 1956, 1997; Brasier 1979; Erwin 1991, 2020; Fortey et al. 1996; Conway Morris 2000, 2006; Elicki 2003; Marshall 2006; Shu 2008; Erwin and Valentine 2013; Zhang and Shu 2014; Shu et al. 2014; Zhang et al. 2014; Briggs 2015; Sperling and Stockey 2018), including two German publications in this journal (Sdzuy 1960; Geyer 1998). Interestingly, the review by Sdzuy (1960) was centered on the question about the poverty of animal fossils in the Precambrian and provided an explanation. The paper was organized more or less in a question-and-answer format. Over the last six decades or more, our knowledge on the nature of the Cambrian Explosion has been growing in many folds through multidisciplinary investigations, and a three-phased pattern and sequence was proposed (Cloud 1948; Shu 2008). However, its cause is still poorly understood and thus was listed as one of the Science’s mysteries in many places. On the basis of currently update knowledge, here we address a current understanding on this unprecedented evolutionary event in the form of questions and answers in order to benefit a broader readership.
What is the Cambrian Explosion?
The Cambrian Explosion initially stemmed from a palaeontological phenomenon, i.e., the relatively sudden appearance of skeletal remains of diverse animals in the lowest known fossiliferous rocks of the Cambrian, which was noted by William Buckland (1786–1856) in 1930s. The notion could be found in his contribution to the famous Bridgewater treatises, the two volumes entitled “Geology and mineralogy considered with reference to natural theology (Pickering, London, 1836)”, which was mentioned in books by Conway Morris (1998) and Levinton (2001). Charles Darwin (1809–1882) thought it a grave difficulty to his theory of evolution and demanded a particular explanation that was hypothetically attributed to imperfection of geological record (Darwin 1859). For quite a long time, the ‘‘missing Precambrian history of animal life’’ stood out as one of the greatest unsolved mysteries in natural science (Schopf 2000). It was Preston Cloud (1912–1991) who alternatively proposed a hypothesis that the diversification of the early Cambrian faunas might be in large part a matter of eruptive evolution in a three-step pattern and sequence: “(1) relatively sudden appearance of marked variability; (2) probable availability and proximity of a variety of ecologic niches; and, finally, (3) increased selective pressure, resulting in the weeding out of inadaptive or poorly adaptive radicles and leading to a more regularly channeled evolutionary phase for tile particular stock involved” (Cloud 1948), which is conformed to the mode of quantum evolution with an explosive phase (Simpson 1944: fig. 35) (Fig. 1). Such a rapid manner of animal diversification during the Precambrian–Cambrian transition was subsequently called Cambrian explosive evolution (e.g., Seilacher 1956), Cambrian Explosion or radiation (Brasier 1979), and Evolution’s big bang (Levinton 1992; Nash 1995). The term “explosion” is favored here for the marked differences from adaptive radiations in the subsequent geological periods (Erwin and Valentine 2013; Zhan 2018).
Extensive and intensive investigations have shown that the Cambrian Explosion is neither a single event nor purely a biological process, but a stepwise evolutionary event of great magnitude that was manifested in many aspects, e.g., burst of animal body plans, increase of body size, acquisition of biomineralized skeletons, substrate revolution, increase of ecosystem complexity, environmental perturbations and so on. All these will be discussed in following sections.
Was the Darwin’s dilemma resolved?
The contrast between Precambrian rocks almost barren of animal fossils and Cambrian rocks in which they may abound had puzzled scientists for over 100 years and was referred to Darwin’s dilemma (e.g., Schopf 2000; Conway Morris 2006). However, Darwin himself inferred “… if the theory be true, it is indisputable that before the lowest Silurian (revised to Cambrian in later editions) stratum was deposited long periods elapsed, as long as, or probably far longer than, the whole interval from the Silurian (Cambrian) age to the present day; and that during these vast periods the world swarmed with living creatures (Darwin 1859: 287).” The discovery of Eozoon canadense, once identified as fossilized shells of giant foraminiferans and later reinterpreted as a pseudofossil (see discussion in Schopf 2000), in the Precambrian Laurentian Formation of Canada was cited by Darwin to support his inference about the existence of living beings long before the Cambrian Period (Darwin 1872). On the one hand, Darwin thought the Cambrian Explosion was inexplicable using his theory and on the other hand he seemed to firmly believe his explanations. This paradoxical combination may lead him draw a conclusion in the end of the Chapter 9 of On the imperfection of geological record, which stated “On this view the difficulties (to his theory of evolution) above discussed are greatly diminished or even disappear (Darwin 1859: 311)”. Taking this case into consideration, it was difficult to know if Darwin really had a dilemma on the Cambrian Explosion.
The hunt for Precambrian fossils has never been ceasing since 1850s. Specimens of Eozoon were first discovered in 1858, a year before publication of Darwin’s theory of evolution, although their biological interpretation finally collapsed. Since then, of course, much has been learnt. It is, however, a very difficult journey! The existence of life before the Cambrian became widely accepted until 1950s (Schopf 2000). Multidisciplinary investigations did extend the life history deep into Archean (e.g., Shen et al. 2001; Duda et al. 2016; Tashiro et al. 2017; Lepot 2020; Mißbach et al. 2021) but only heightened the sharpness of the beginnings of animal fossils across the Ediacaran–Cambrian transition. All fossil records still point to an explosion of animal life near the beginning of the Cambrian (Levinton 1992), please see discussions below.
What does the fossil record tell us?
Precambrian palaeontologists are at loss to search for animal remains in ancient rocks, but results are somewhat awkward. The findings do not extend the dawn of animal life much further back. Body, trace, and chemical remains of animals are not as ancient as Darwin predicted but have been found in the time slice just before the Cambrian, i.e., late Ediacaran (~ 580.0–538.8 Ma). Moreover, most Ediacaran candidates of animals are a bit weird and thus difficult to be placed in any known animal group. More familiar animals arrived in the early Cambrian.
Body fossils
The Ediacaran was far from lifeless, but it was a difficult world! The most impressive fossil record is the three Ediacara-type macrofossil assemblages, termed the Avalon, White Sea, and Nama, occurring in loosely ascending stratigraphic order (Waggoner 2003; Xiao and Laflamme 2009; Boag et al. 2016). Early workers interpreted these soft-bodied organisms as ancestors of later animal phyla based on their symmetry and general appearance (e.g., Glaessner 1984), which appeared to undermine the importance of the Cambrian Explosion. On the contrary, Pflug (1972, 1974) interpreted some forms from the Nama assemblages as colonial organisms (Petalonamae) unrelated to modern animals. Seilacher (1989, 1992) went even further to propose that the bulk of the Ediacara-type fossils were representing an extinct kingdom, the Vendobionta, on the basis of their shared characters: foliate bodies, quilted construction, lack of organs, and occurring as impressions. His assignments were later modified by himself (Seilacher 2007). The core members of vendobionts were placed into an extinct group allied to rhizpodean protists such as the Xenophyophoria as originally suggested by Zhuravlev (1993). The vendobiont hypothesis implied that the Cambrian Explosion was accompanied with a major extinction event, which re-established the importance of the Cambrian Explosion (Seilacher 1997). Now most Ediacaran workers tend to consider Ediacarans as a grab bag of disparate life forms (Watson 2020) rather than treat them as a homogeneous group. A couple of vendobionts and some newly discovered forms were interpreted as different lineages within the Metazoa (Watson 2020), e.g., Dickinsonia as a ctenophore (Zhang and Reitner 2006) or placozoan (Sperling and Vinther 2010), Haootia as a cnidarian (Liu et al. 2014a, b), Kimberella as a mollusk (Fedonkin and Waggoner 1997), and Yilingia as an annelid or arthropod (Chen et al. 2019). Accordingly, the prominent status of the Cambrian Explosion in life history was questioned again by some researchers (see Watson 2020). However, such placements were mostly based on general resemblance rather than shared diagnostic features. These forms are difficult to relate to living phyla and it is hard to find decedents for them in Cambrian faunas. They were Ediacarans and died off at the end of the Ediacaran. The majority of Cambrian faunas are newcomers.
Of course, there are other taphonomic windows that contain different body designs. Notably, microfossil assemblages from the Ediacaran phophatic deposits (Xiao and Knoll 2000; Zhang and Zhang 2017) yield abundant spherical fossils that demonstrate patterns of cell adhesion similar to animals (Yin et al. 2019) but they are not necessarily metazoan embryos (Zhang and Zhang 2017; Erwin 2020). Additionally, Ediacaran fossils contain abundant and diverse organisms with a tubular morphology (Fig. 2), including forms either fully soft-bodied or lightly mineralized (Cai et al. 2013; Schiffbauer 2016; Schiffbauer et al. 2016). The tubes are not confined to any particular facies but can be present in both siliciclastic and carbonate rocks. Ediacaran tubes were frequently compared to metazoans with a tubular construction. However, such a simple constructional morphology is utilized today by a variety of multicellular organisms from disparate kingdoms and hence these tubes may very well be a polyphyletic group (Droser et al. 2017). Most Ediacaran tubes went extinct near the Ediacaran–Cambrian boundary though a couple of taxa have a stratigraphical distribution overlapping the lower range of the earliest small shelly fossils of the Cambrian (Yang et al. 2016; Zhu et al. 2017; Cai et al. 2019).
The Cambrian was a refreshed world! Everything looked new to Ediacarans and was much easier to understand. Most living animal phyla made their first appearance in the fossil record during the first 20 million years of the Cambrian Period (Erwin et al. 2011; Zhang and Shu 2014; Shu et al. 2014). Most fossils can be comfortably placed into a stem or crown group of a living phylum. Apart from small shelly fossils that are skeletal remains of many animal lineages, soft-bodied worm-like animal reaching 14 mm wide and 25 cm long was discovered from a sandstone surface of the lowest Cambrian (Zhang et al. 2017a). The preservation is similar to Ediacara-type biotas, which implies that this type of taphonomic window persists into the earliest Cambrian. Many more soft-bodied forms were found in Burgess Shale-type biotas, such as Chengjiang and Qingjiang (Hou et al. 2017; Fu et al. 2019). The Fortunian (ca 533.8–529.0 Ma) phosphorous deposits of the Kuanchuanpu Formation yield diverse of small shelly fossils along with soft-bodied meiofaunas (Bengtson and Yue 1997; Liu et al. 2014a, b). In contrast, there is no unambiguous evidence for metazoans among Ediacaran phosphorous deposits, e.g., the Weng’an biota (Xiao and Knoll 2000) and the Zhenba microfossil assemblage (Zhang and Zhang 2017), though fossils are super abundant. Remarkably similar taphonomic windows yield quite different biotas, which again manifests the power of the Cambrian Explosion (Bottjer et al. 2020).
Trace fossils
The trace-fossil record is continuous through the Ediacaran–Cambrian transition and supports an explosive evolution (Seilacher 1956, 1999; Jensen 2003; Jensen et al. 2005; Mángano and Buatois 2014, 2016). A four-phase evolutionary model was recently proposed (Mángano and Buatois 2020). Late Ediacaran phase 1 is represented by very simple, unbranched trails and shallow burrows (Fig. 2), which demonstrates the presence of motile benthic bilaterians in the Ediacaran; phase 2 (broadly correlated to the Nama Assemblage of the latest Ediacaran) demonstrates a moderate intensity of bioturbation as evidenced by the appearance of more complex and penetrative burrows, bulldozing traces, and drilling predation; phase 3 (the Fortunian, ~ 538.8–529.0 Ma) is characterized by an unparalleled increase in the number of trace-fossil morphologies, essentially providing overwhelming evidence of a rapid diversification of body plans; phase 4 (~ 529–509 Ma) reveals an increase in depth and extent of bioturbation and a renewed diversification at ichnogenus level. Although simple traces of bilateral animals were present by 560 Ma (Jensen et al. 2005) or early (Pecoits et al. 2012) and a possible trackway of a bilaterian with paired appendages was found in the latest Ediacaran (Chen et al. 2018), the full suite of metazoan trace fossils that reflect a rapid diversification of body plans does not appear across a broad range of marine environments until close to the earliest Cambrian (Mángano and Buatois 2020).
Chemofossils
Chemofossils, also known as molecular fossils or biological marker compounds (biomarkers for short), are chemical remains of ancient organisms that are either directly extracted from fossil remains or isolated within sedimentary rocks. They are a powerful complement to fossil remains in revealing the history of life (Briggs and Summons 2014). However, their utility in tracing the history of early metazoans is greatly diminished because most biologically informative molecules are not able to survive geological alterations for millions of years (Briggs and Summons 2014: table 1) and geologically durable biomarkers that are diagnostic for metazoan clades are rarely known to science. Two proposed demosponge biomarkers, 24-isopropylchoestane and 26-methylstigmastane, co-occur continuously through the ~ 660–540 Ma old marine sequences of the South Oman Salt Basin but are rare or absent in samples younger than the Cambrian (Love et al. 2009; Zumberge et al. 2018). If they were validated the molecular record of sponges would be more than 100 Ma prior to the earliest record of siliceous spicules (Antcliffe et al. 2014). However, sponge origin of the supposed biomarkers has been challenged by a number of studies (e.g., Antcliffe 2013; Botting and Muir 2018; Botting and Nettersheim 2018; Nettersheim et al. 2019; Bobrovskiy et al. 2020; van Maldegem et al. 2020; Love and Zumberge 2021). In addition, coprostane was proposed as a metazoan biomarker and detected in the iconic Ediacaran fossil Dickinsonia (Bobrovskiy et al. 2018). Coprostane has been known to be produced via the bacterial reduction of steroid coprostanol in the gut of higher mammals, unstable on geological time scales, and absent in much younger, exceptionally preserved animal fossils (Summons and Erwin 2018). Its presence in Dickinsonia was unusual and hence led to a hypothesis of distinct metabolic physiology (Summons and Erwin 2018) and a suggestion for additional work to exclude the possibility of exogenous origin (Love and Zumberge 2021). The steroids from Dickinsonia specimens were almost exclusively composed of compounds with 27 carbon atoms (cholesteroids), which were thought to be a signature of all animal phyla other than sponges and a few mollusk taxa (Bobrovskiy et al. 2018; Summons and Erwin 2018). In fact, cholesterol can be a sterol of some protists (Boëchat et al. 2007; Kodner et al. 2008) and many algae (Govindan et al. 1993).
Summary
Chemofossil record provides suggestive but not conclusive evidence for the presence of demosponges in pre-Ediacaran rocks. The trace-fossil record indicates trace makers of bilateral animals were in place by 560 Ma, generally coinciding with the divergence of ecdysozoans and lophotrochozoans estimated based on molecular studies (Fig. 3). This in turn suggests that non-bilateral clades (sponges, placozoans, cnidarians and ctenophores) branched earlier than this date in the animal tree (Fig. 3). However, it is curious that unequivocal sponge fossils are generally absent in the Ediacaran (Sperling et al. 2010; Antcliffe et al. 2014). Ediacaran body fossils may contain early representatives of metazoans. However, they generally lack diagnostic features of metazoans and thus are difficulty to link to Cambrian faunas or living phyla. It is likely that during the Ediacaran Period the ancestors of modern marine animals lived alongside Ediacarans (e.g., vendobionts) until the latter went extinct (Gould 1989; Briggs 2015). Both body and trace fossils support an explosive evolution. Most bilateral clades appeared during the first 20 million years of the Cambrian Period. Exceptionally preserved Burgess Shale-type fossil Lagerstätten, e.g., Chengjiang and Qingjiang biotas, indicate that almost all major metazoan clades, including vertebrates, appeared by 518 Ma.
Timeline and evolutionary dynamics of Gene Regulatory Networks (GRNs) against holozoan phylogeny based on studies cited in the text. Dashed lines represent lineages with unstable phylogenetic positions. Later acquisition of lineage-specific genes and/or extensive co-option of existing regulatory components many have contributed to the rapid diversification of animals during the Cambrian explosion
When did animals originate?
It is certain that animal phyla did not originate precisely when we first find them. In other words, the earliest fossil animals yet discovered may not be the very first animals ever to have existed. We cannot be sure how early each clade arose prior to its earliest fossil evidence. Fossil first appearances of animal phyla provide a conservative minimum time estimate for the origins of animal clades (Zhang and Shu 2014). However, fossils are critical to calibrating the age of branching events in a phylogeny and, by extrapolation, to computing a molecular clock. Origins of metazoan clades estimated by molecular clock studies were summarized in Erwin (2020) as the following: the divergence of Metazoa from choanoflagellates is earlier than 900 Ma; the last common ancestor of living animals lies at about 750 Ma; the origin of the Eumetazoa was about 640 Ma; the Bilateria spilt into the Protostomia and the Deuterostomia at ~ 630 Ma; deuterostomes diversified at ~ 580 Ma; lophotrochozoans diverged from ecdysozoans about 600 Ma and both supergroups further diversified at ~ 560 Ma (Fig. 3).
Molecular timescales for the origin of phyla are relatively poor in precision and usually have startlingly large error ranges (dos Reis et al. 2015: fig. 2). For instance, the Metazoa was estimated to originate between 833 and 650 Ma, with the younger limit close to the earliest record of sponge biomarker. Pisani and Liu (2015) argued that the poor precision may only be overcome by improving our knowledge of the fossil record and fossil-free molecular divergence times were meaningless in deep time. Interestingly, molecular clock estimates for the divergences of crown groups with skeletonized body parts, such as Euarthropoda and Brachiopoda, consistent with their earliest fossil appearances (Erwin et al. 2011; Erwin 2020). Therefore, the earliest record of animal skeletons (Fig. 4) did likely capture their originations and thus provided robust calibrations for refining the molecular timescale.
Fossil record of mineralized skeletons showing a three-phased biomineralization of metazoan clades. Vertical bars representing mineralogy and stratigraphical ranges of animals with mineralized skeletons. Time scales are based on Peng et al. (2020). Geological record and mineralogy of mineralized taxa are based on the following references: Cloudina from Yang et al. (2016, 2020), Zhu et al. (2017), Cai et al. (2019); Namapoikia from Wood et al. (2002), Wood and Zhuravlev (2012), Wood and Penny (2018); Namacalathus from Grotzinger et al. (2000) and Wood (2011); Sinotubulites from Chen et al. (2007), Wood and Zhuravlev (2012); Anabarites from Kouchinsky and Bengtson (2002), Yang et al. (2016); Protoconulariids from Kouchinsky et al. (2012); Chancelloriida from Khomentovsky and Karlova (2005), Yun et al. (2021); Hexactinellida from Rigby (1986), Wood and Zhuravlev (2012), and Murdock (2020); Demospongiae from Bengtson et al. (1990), Müller et al. (2008), Wood and Zhuravlev (2012), and Murdock (2020); Calcarea from Kruse et al. (1995), Reitner and Mehl (1995, 1996), Wood and Zhuravlev (2012), Murdock (2020); Archaeocyatha from Rozanov and Zhuravlev (1992), Wood and Zhuravlev (2012); Anthozoa from Tynan (1983), Sorauf and Savarese (1995); Protoconodonta from Khomentovsky and Karlova (2005), McIlroy and Szaniawski (2010); Halwaxiida from Conway Morris and Peel (1990), Vinther and Nielsen (2005); Hyolitha from Vinn (2006), Kouchinsky (2001), Skovsted and Peel (2011); Cambroclavida from Conway Morris et al. (1997), Steiner et al. (2007); Paracarinachitida from Conway Morris and Chen (1991), Wood and Zhuravlev (2012); Tianzhushanellidae from Kouchinsky et al. (2012); Helcionelloida from Khomentovsky and Karlova (2005), and Wood and Zhuravlev (2012); Polyplacophora from Carter and Hall (1990), Treves et al. (2003), Hoare and Pojeta (2006); Scaphopoda from Peel (2006), Murdock (2020); Stenothecoida from Rozanov and Zhuravlev (1992), Ushatinskaya and Zhuravlev (1994), Wood and Zhuravlev (2012); Rostroconchina from Kouchinsky (2000), Khomentovsky and Karlova (2005), Murdock (2020); Bivalvia from Runnegar and Bentley (1983), Runnegar (1985); Gastropoda from Parkhaev (2017), Murdock (2020); Cephalopoda from Chen and Teichert (1983), Landing and Kröger (2009); Tommotiida from Khomentovsky and Karlova (2005), Skovsted et al. (2008); Mobergellidae from Rozanov and Zhuravlev (1992), Skovsted (2003), Topper and Skovsted (2017); Linguliforma from Skovsted and Holmer (2003), Khomentovsky and Karlova (2005), Murdock (2020); Rhynchonelliformea from Ushatinskaya and Zhuravlev (1994), Ushatinskaya and Malakhovskaya (2006); Bryozoa from Taylor and Weedon (2000), Landing et al. (2010), Taylor et al. (2013); Serpulidae from Kupriyanova et al. (2010); Palaeoscolecida from Han et al. (2007), and Kouchinsky et al. (2012); Lobopodia from Kouchinsky et al. (2012); Bradoriida from Liu et al. (2008), Kouchinsky et al. (2012); Phosphatocopida from Müller (1979), Kouchinsky et al. (2012); Aglaspidida from Briggs and Fortey (1982); Pytophillaspis from Lin et al. (2010); Trilobita from Teigler and Towe (1975), Zhang et al. (2017b); Cirripedia from Lowenstam and Weiner (1992); Decapoda from Bentov et al. (2016a,b); Isopoda from Vittori et al. (2016); Stomatopoda from Bentov et al. (2012); Currey et al. (1982); Ostracoda from Bate and East (1972); Echinodermata from Rozanov and Zhuravlev (1992), Guensburg and Sprinkle (2001); Calcichordata from Dominguez et al. (2002), Lefebvre (2007); Conodonta from Bengtson (1983), Miller (1984); Ascidiacea from Lowenstam and Abbott (1975), Chen et al. (2003); Vertebrata from Rücklin et al. (2012)
In addition, molecular estimates that might overstate clade ages (Budd and Mann 2020) point to the origin of metazoans and bilaterians tens to hundreds of millions of years earlier than their first appearances in the fossil record (Wray 2015), which led to a suggestion that the Cambrian Explosion is a phenomenon of fossilization, while biological diversity was established in the Neoproterozoic (dos Reis et al. 2015). Therefore, molecular estimates revived the longstanding conundrum of the Cambrian—whether the first animal fossils faithfully reflect an explosion in animal biodiversity or merely an explosion of fossils (Runnegar 1982; Conway Morris 2006). Please see the question below.
Is the Cambrian Explosion a genuine evolutionary event or an artifact?
It is an incontestable fact that fossils of most animal lineages made their first appearance asynchronously in a geologically short period of time during the Ediacaran–Cambrian transition (Erwin et al. 2011; Zhang and Shu 2014; Zhang and Cui 2016), which was frequently interpreted as a taphonomic artifact since Darwin time (e.g., Darwin 1859; Runnegar 1982; Fortey et al. 1996; dos Reis et al. 2015). Fortunately, much has been learned in the last 160 years, not least multiple discoveries of Cambrian and Precambrian soft-bodied fossil Lagerstätten. Multiple sources of evidence are strongly suggestive of a real evolutionary event being recorded rather than an artifact of an imperfect fossil record (Conway Morris 1998, 2000, 2006; Mángano and Buatois 2014, 2016, 2020; Briggs 2015), e.g., rapid diversification of animals coincided with the evolution of biomineralized shells (Briggs 2015), continuous record of trace fossils (Mángano and Buatois 2020), and the apparent orderly appearance of taxa in the Ediacaran to Cambrian (Zhang and Shu 2014; Zhang and Cui 2016; Budd and Mann 2020). The combination of the body and trace-fossil record demonstrates a progressive diversification through the end of the Neoproterozoic well into the Cambrian (Budd and Jensen 2000). The nature of the Cambrian Explosion is manifested in multiple aspects, which will be discussed below.
The earliest uncontroversial records of bilaterian activity reflected by trace fossils are approximately 560–555 Ma (Jensen 2003; Jensen et al. 2005; Mángano and Buatois 2020), which provide a critical constraint on the timing of appearance of large worm-like animals capable of creeping and/or shallowly burrowing. Phylogenetic affinity of the trace makers is difficult to be determined because a range of bilaterian lineages exploit a worm-like body plan. Since worm-like bilaterians were in place by 560 Ma, it is self-evident that pre-bilateral lineages (Porifera, Placozoa, Cnidaria and Ctenophora) must diverge successively from the main branch of the animal tree before this age. The absence of convinced metazoan fossils earlier than 560 Ma, together with sponge biomarker and molecular estimates (see above), strongly implies a cryptic evolution of early metazoans, which was supposed to be temporally distant to the abrupt increase in diversity and disparity during the Ediacaran–Cambrian transition—the Cambrian Explosion in the strict sense (Smith and Harper 2013). It appears reasonable to assume that these early animals were small and incapable of leaving preservable marks (Valentine and Erwin 1987; Erwin 2020; but see Budd and Mann 2020). However, Daley et al. (2018) argued that taphonomic conditions of the Ediacaran were no inferior to the Cambrian and soft-bodied organisms, either small or large, were found in a range of taphonomic windows of the Ediacaran Period. Yet, undoubted metazoan fossils have not been recovered from rocks earlier than 560 Ma old trace fossils, which raise a possibility that they might be extremely rare or ecologically negligible. Alternatively, it was assumed that original members of the clade may have had different characteristics from those defining the crown group (Erwin 2020). The earlier origin of the Metazoa and the divergence of basal clades were considered as a distinct event (Smith and Harper 2013), which are effectively decoupled from the later increases in body size, acquisition of characteristic body plans and (in some clades) skeletonization as seen across many lineages during the Cambrian Explosion (Erwin et al. 2011; Erwin and Valentine 2013; Sperling and Stockey 2018).
What is the nature of the Cambrian Explosion?
Explosion of fossils resulted from explosion of animals! Fossil record does capture the explosive evolution of animal body plans along with biomineralization, body size variation and increase of ecosystem complexity. Let us come back to the fossil record again and look at the nature of the Cambrian Explosion.
Three-phased explosion of body plans
As discussed above, the earliest trace fossils strongly suggest the presence of vermiform bilaterians in the late Ediacaran. However, a major burst of bilateral body plans took place in the first 20 Ma of the Cambrian (Erwin and Valentine 2013; Zhang and Shu 2014; Mángano and Buatois 2020). Taking the Euarthropoda, the most diversified phylum, for example, molecular clock studies constrained the origin between 561 and 530 Ma (Rota-Stabelli et al. 2013; dos Reis et al. 2015; Lozano-Fernandez et al. 2016), spanning into the Cambrian. The earliest arthropod trace dates to the Fortunian (~ 538.8–529.0 Ma) (Mángano and Buatois 2020). In addition, divergence time analyses using morphological data also recover a Cambrian origin for the Euarthropoda (Wolfe 2017). The estimated timing of origin is remarkably congruent with the fossil record (Daley et al. 2018).
Within the Bilateria, both ecdysozoan and lophotrochozoan lineages of the Protostomia were well represented in the Terreneuvian Epoch (~ 538.8–521.0 Ma), while deuterostome lineages did not appear in the fossil record until the Cambrian Stage 3 (~ 521.0–514.5 Ma). The basal metazoan clades blow the node of the Bilateria ought to be in place in the Ediacaran Period though unequivocal fossils remain to be found. In contrast, basal metazoan clades were well represented in the early Cambrian. Their diversification was in parallel to that of bilaterians.
In this regard, a three-phased model of the Cambrian Explosion was suggested to successively give rise to the three subkingdoms of the Metazoa, i.e., Diploblasta (basal metazoans), Protostomia and Deuterostomia, respectively (Shu 2008; Shu et al. 2014), which ultimately led to the formation of a complete animal tree and hence concluded the Cambrian explosion (Shu et al. 2014; Shu and Han 2020). The three-phased manner appear to coincide with episodic patterns of trace and skeletal fossil records (Mángano and Buatois 2014; Fig. 4). The model was largely based on first fossil appearances of metazoan lineages and thus may vary with emerging discoveries (Zhang and Shu 2014). More recently, a possible trackway of a bilaterian with paired appendages was reported from the late Ediacaran Shibantan biota (Chen et al. 2018) and a number of putative deuterostomes were recovered from the Fortunian Stage, e.g., meiofaunal deuterostomes (Han et al. 2017; reconsidered as a primitive scalidophoran or the nearest common ancestor of the Bilateria in Shu and Han 2020), a stem group echinoderm (Topper et al. 2019, 2020; but see Zamora et al. 2020), and enteropneust hemichordates (Maletz 2019). These discoveries are critical to constrain the duration of the Cambrian Explosion and hence remain to be tested and verified.
Crown groups along with numbers of stem groups
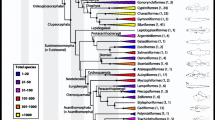
The Cambrian Explosion produced a rich fauna with or without sclerotized body parts. Both skeletonized and soft-bodied faunas contained numbers of weird forms whose morphologies do not fit easily within living phyla or classes (Valentine 2004) and hence they were traditionally placed in separate phyla or classed. The application of stem- and crown-group concepts assigned most of these weird forms to stem groups of a high-rank crown clade, e.g., a class, a phylum or a subkingdom (Budd and Jensen 2000). Most phylum-level clades of the Metazoa must have been present, at least as stem groups, by the time of the Chengjiang biota (~ 518 Ma), though a number of living phyla are absent in the fossil record. There are a number of cases that crown groups had appeared at the class level, e.g., Linguilida among Brachiopoda, Bivalvia among Mollusca, and Pancrustacea among Euarthropoda (Erwin and Valentine 2013). However, there might have many more stem groups with a high-rank phylogenetic position. For example, the superphylum Panarthropoda comprises three living phylum-level clades, Tardigrada, Onychophora and Euarthropoda. Many of Cambrian forms (e.g., Anomalocaris, Opabinia and others) were comfortably placed between the three living phyla (Daley et al. 2018). Each of these weird forms is phylogenetically equivalent to or above the living crown groups and was previously thought to represent a phylum-level body plan (e.g., Whittington 1979; Gould 1989). Some may have a temporary success of diversity and longer evolutionary history, e.g., radiodonts, known from Cambrian to Devonian, represented by 17 genera and 28 species(Wu et al. 2021a,b), and others are probably ephemeral orphans, e.g., Opabinia (known only in Burgess Shale). Therefore, it is a prominent feature that the Cambrian was a time of crown groups nested by many stem groups with a high-rank taxonomy of Linnaean system (classes and above).
Broad disparity
Disparity is the diversity of animal forms or body plans, which can be measured by use of Linnaean ranks and a variety of quantitative approaches (Erwin 2007). Each body plan or a high-rank clade, e.g., a phylum, has a set of distinctive features. It has long been recognized that in no case is a morphological continuum found across a broad range of body plan morphologies nor do phyla resemble each other more closely during their early fossil histories (Valentine 2004). Simply there are morphological gaps between phyla. By analyzing the timing of appearance of Linnaean rank taxa in the fossil record, paleontologists realized that the limits on animal disparity were early in animal evolutionary history (e.g., Erwin et al. 1987). By the reinterpretation of the Burgess Shale fauna, Gould (1989: fig. 3.72) suggested a pattern of rapid, maximal disparity in the early history followed by later removal of most groups (stem groups) by extinction that leaves large morphological gaps among high-rank clades. This pattern is applicable to high-rank clades and metazoans as a whole. Particularly, he argued that that the morphological disparity of arthropods at a single locality (Burgess Shale) surpassed all extant arthropods, which inspired considerable efforts to understand disparity. Subsequent quantitative studies have shown that most clades achieved their maximal disparity (or morphological breadth) during a short time interval close to their first appearance in the fossil record in the early Cambrian (see a review in Erwin 2007; Hughes et al. 2013). A more recent study by mapping of fossil and living metazoan morphospace demonstrated that the majority of phylum-level clades achieved maximal initial disparity in the Cambrian and that the overall disparity was already very broad in the early history of animal evolution, although the envelope of disparity explored by the Metazoa has increased through geological time (Deline et al. 2018). It is worth mentioning that new discoveries of weird forms in Cambrian deposits would increase the morphological breadth of Cambrian animals (e.g., Zeng et al. 2020).
What are causes of disparity? Morphological discreteness among animal clades can be depicted as clumpy occupation of morphospace. A number of views have been offered to explain the clumpiness of metazoan morphospace occupation, i.e., (1) morphological continuum broken up by subsequent extinction, (2) incomplete exploration of morphospace, (3) contingent occupation and elimination, (4) fitness landscapes, and (5) developmental constraints (see a summary in Erwin 2007). These explanations are partially overlapping and may not be mutually exclusive. Recent analyses including fossils and phylogenetic ancestors indicated that the majority of the empirical morphospace has not been explored in metazoan evolutionary history and the morphological discreteness of modern clades is largely a consequence of the extinction of phylogenetic intermediates (stem groups) (Deline et al. 2018).
Increase of generic diversity
Fossil first appearances of animal genera during the Terreneuvian occurred in three pulses; a small pulse in the early Fortunian, a large one in the late Fortunian, and a moderate increase in the Cambrian Stage 2 (Maloof et al. 2010: fig. 2). Global data in the later stages of the Cambrian are currently not yet available. The pattern of South China shows a different pace, a jump from 11 genera of Small Shelly Fossils (SSFs) in Fortunian to 169 in Stage 2. Stage 3 has the highest diversity (431 genera or more) largely for the contribution of the Chengjiang biota (Li et al. 2007 recompiled by Erwin and Valentine 2013). Up till now, more than 280 species have been described from the Chengjiang biota (Zhu et al. 2019).
Similarly, the increase of ichnodiversity took place in pulses rather than at a steady pace. About ten ichnogenera have been known in Ediacaran. A big jump of 433% increase during the Terraneuvian was followed by a moderate increase of ~ 40% ichnogenera, which is obviously attributed to the Cambrian Explosion and unparalleled in the rest of the Phanerozoic, emphasizing the uniqueness of this event (Buatois and Mángano 2018; Mángano and Buatois 2020). The pattern of ichnodisparity is well coordinated with that of ichnodiversity (Mángano and Buatois 2020: fig. 5). The two pulses of diversity increase separate the Ediacaran–Cambrian transition into three phases, late Ediacaran, Terreneuvian, and Cambrian Stage 3, respectively (Mángano and Buatois 2014).
Acquisition of mineralized skeletons
Fossil first appearances of metazoan lineages with mineralized skeletons have interesting points. Two-phased metazoan biomineralization was previously proposed, i.e., a fast evolutionary radiation of the Lophotrochozoa during the Terreneuvian followed by radiation of the sclerotized ecdysozoans during Stage 3 (e.g., Kouchinsky et al. 2012). However, there are a number of facts worth a discussion. First, appearances of mineralized lophotrochozoans in the Terreneuvian were preceded by a number of tubular fossils with uncertain affinities (e.g., Cloudina, Sinotubulites, Namacalathus, and Arnabarites) and a modular form Namapoikia spanning from ~ 550–539 Ma (Fig. 4). The tubular forms have long been recognized as mineralized metazoans thought their phylogenetic affinities remain ambiguous, while Namapoikia was interpreted as an encrusting poriferan (Wood and Penny 2018). Second, the Terreneuvian is still a long period, lasting ~ 18 million years and recorded asynchronous appearances of mineralized lophotrochozoans along with numbers of other clades (Kouchinsky et al. 2012). Third, the Terreneuvian biomineralization can be further subdivided into two pulses: a group of lophotrochozoans (e.g., Halwaxiids, hyoliths, and molluscs) alongside siliceous sponges, chancelloriids, and protoconulariids became mineralized during the Fortunian (~ 538.8–529.0 Ma), while another group of lophotrochozoans (brachiopods, tommotiids, and stenothecoids) accompanied by calcarean sponges, archaeocyaths and corallomorphs was calcified during the Cambrian Stage 2 (~ 529–521 Ma) (Fig. 4). The Cambrian Stage 3 is featured by an abrupt widespread mineralization of ecdysozoans accompanied by echinoderms (Fig. 4).
Overall, the biomineralization through the Ediacaran–Cambrian transition is a three-phase process (Fig. 4). Each phase crosses a range of lineages. Phase 1 (~ 550.0–538.8 Ma) is characterized by calcareous tubes and modular structures. Phase 2 (~ 538.8–521.0 Ma) is the most extensive mineralization event, represented by a wide-range of lophotrochozoan and non-bilaterian metazoan clades, which appeared in two pulses. Phase 3 (~ 521.0–514.5 Ma) is featured by a widespread mineralization of ecdysozoans along with echinoderms.
Fluctuations of seawater chemistry, e.g., Magnesium/Calcium ratio, were frequently invoked to explain the changing skeletal mineralogy from aragonite to calcite (e.g., Porter 2007; Zhuravlev and Wood 2008). The turning point is the prominent negative excursion of carbon isotopic record in the middle of Stage 2, involving the first appearances of high-magnesium skeletons and a decrease of the Ma/Ca ratio. However, biomineralization is an enzymically controlled process that is different from the abiotic one in multiple aspects (Degens 1976; Degens et al. 1986; Weiner and Dove 2003; Jackson et al. 2007), and the mineralogical evolution of skeletal lineages did not follow the pace of the seawater Magnesium/Calcium ratio through time (Fig. 4). In addition, biocalcification was proposed as a detoxification mechanism because the calcium ion is pharmacologically one of the most disruptive substances for normal cell functions (Simkiss 1977). Accordingly, the widespread biomineralization across metazoan lineages was considered as a response to the increase of toxic Ca2+ level in the ocean during the Precambrian–Cambrian transition (Kazmierczak et al. 1985; Degens et al. 1985, 1986). It should be noted that the fossil record indicates that numbers of entirely soft-bodied animals lived alongside those with mineralized skeletons though Ca2+ concentration of the seawater was increasing during the Cambrian Explosion (Arp et al. 2001; Lowenstein et al. 2001, 2003), which suggests a detoxification of Ca2+ threat other than biocalcification. Furthermore, it is difficult for seawater chemistry hypotheses to explain the timing of mineralization in a specific lineage and successive appearances of phosphatic skeletons from distantly related lineages. Notably, why did the sudden mineralization of ecdysozoans preceded by a widespread biomineralization of lophotrochozoans? The seawater chemistry provides cations and anions that are prerequisites for animal making skeletons, but it alone is insufficient to drive biomineralization during the Ediacaran–Cambrian transition. Alternatively, biomineralization among animal lineages has long been considered as an escalating defensive response driven by increasing predation pressure (e.g., Vermeij 1990), while the advent of microphagous predators postdates the initial episode of biomineralization and requires a trigger as well (Zhang and Shu 2014; Zhang et al. 2014). Finally, or intrinsically, metazoan lineages separately acquired the ability to form biomineralized skeletons through lateral gene transfer of mineral-forming gene toolkits during the Cambrian Explosion (Jackson et al. 2011) or biomineralization is genetically a deep homology that was independently co-opted in different lineages in different time (Erwin 2020; Murdock 2020).
Increase of body size
The maximum body volume of organisms preserved in the fossil record has increased by ~ 16 orders of magnitude over the last 3.5 billion years, demonstrating two pronounced ~ 6-ordered jumps of approximately equal magnitude in the mid-Paleoproterozoic (~ 1.9 Ga) and during the Ediacaran through Ordovician (~ 635.0–443.8 Ma), respectively. Each size step required a major innovation in organismal complexity—first, the eukaryotic cell and later, eukaryotic multicellularity, and corresponded to an oxygenation period (Payne et al. 2009).
The second size increase was manifested by appearances of large complex multicellular organisms (e.g., algae, vendobionts, tubular forms, and metazoans) and the duration embraces the Cambrian Explosion. The earliest Cambrian was characterized by Small Shelly Fossils but there were examples of Fortunian fossils (Zhang et al. 2017a; Marusin and Grazhdankin 2018) comparable to the body size of Ediacara-type fossils. The large size was reached before the Cambrian Explosion. A quantitative study of skeletal species from Siberia demonstrated marked changes in maximum linear body size dimensions but no unidirectional trend throughout the early Cambrian (Zhuravlev and Wood 2020). However, the variation of the linear body size was in interesting paces: the size of archaeocyath, hyolith, and helcionelloid mollusc species varied in a synchronous rhythm over million-year timescales, with peaks positively correlated with origination rate and total species diversity, which appear to be a consequence of external drivers. The response of brachiopods is quite different, calcitic shells showing a general increase in body size while phosphatic shells decreasing in size through time (Zhuravlev and Wood 2020).
Increase of ecosystem complexity
Microbially dominated ecosystems appeared billions of years before the Cambrian Explosion and persisted into the earliest Cambrian (e.g., Buatois et al. 2014; Duda et al. 2016). The modern-style, metazoan-dominated ecosystems are essentially the outcome of the Cambrian Explosion (Erwin and Tweedt 2012; Zhang and Shu 2014). Ecological networks expanded considerably in both size and complexity between the Ediacaran and the early Cambrian as measured by the number of life modes (Bambach et al. 2007; Bush et al. 2011; Hua et al. 2020), complexity of food web (Dunne et al. 2008), and ecosystem engineering (Erwin and Tweedt 2012; Mángano and Buatois 2014). A total of 12 different life modes were recognized in Ediacaran macrofossil assemblages (Avalon, White Sea, and Nama) and 30 in Chengjiang and Burgess Shale biotas (Bambach et al. 2007), with a gain of 24 and a loss of 6 life modes through the Ediacaran–Cambrian transition. Relatively, a minor positive ecological feedback during the Ediacaran was followed by a substantial increase during the early Cambrian, principally through bioturbation and the appearance of a number of structural engineers (Erwin and Tweedt 2012); a major shift in benthic ecological structure (agronomic revolution) resulted in the establishment of the Phanerozoic-style ecology during the Cambrian Stage 2 (~ 529–521 Ma), which in turn drove a further diversification of deposit-feeding strategies by Cambrian Stage 3 (~ 521.0–514.5 Ma), favoring an ecological spillover scenario (Mángano and Buatois 2014). The fundamental structure of metazoan marine food webs appears to have been established by the Cambrian Stage 3 as seen in the Chengjiang biota (~ 518 Ma).
How long did the Cambrian Explosion last?
To answer this question, we have first to define the opening and closure of this unprecedented evolutionary event. It is generally accepted that the arrival of most metazoan body plans must mark the end of the Cambrian Explosion, which was followed by broad-scale evolutionary stasis throughout the remainder of the Phanerozoic (Gould 1989; Paterson et al. 2019; Erwin 2020; Shu and Han 2020). If so, the first appearance of trilobite (~ 521 Ma) is a good marker. By the arrival of trilobites, or about 18 million years into the Cambrian Period, the great burst of evolutionary novelty and innovation transitioned to more traditional dynamics of speciation and extinction with fewer morphological novelties (e.g., Paterson et al. 2019). The opening of the event may not exactly coincide with the Ediacaran–Cambrian boundary (~ 538.8 Ma). Metazoans may have appeared more than ~ 750–660 million years ago as suggested by molecular and biomarker studies, well before the Cambrian. The earliest physical remains of definitely metazoan origin are traces of bilateral animals about 560 million years ago, which can be used as a minimal calibrating time for the origin of bilaterians. Non-bilaterian clades must have successively diverged from the main branch of animal phylogeny before this age. However, they were ecologically negligible, or their diversification might be later in the Ediacaran–Cambrian transition for the paucity of physical remains in much earlier strata. Ecologically it took metazoans more than 100 million years to take over the ocean, from their emergence in the Cryogenian ( ~ 720–635) or earlier, through a long silent period, becoming ecologically non-negligible by 560 Ma, further through an ecological revolution during the first 18 Ma of the Cambrian, and finally forming the metazoan-dominated ecosystem by 521 Ma. Fossil record also indicates that most metazoan body plans appeared after 560 Ma. Therefore, the maximal duration of the Cambrian Explosion is about 40 Ma. The episodic biomineralization across a range of animal lineages was embraced in this time window (Fig. 4).
Forty million years is rapid in the larger scheme of Earth’s history, just about 1% of life’s history. The Cambrian Explosion is a multifold event, involving morphological innovations, skeletonization, and ecological revolution. Most body plans emerged within this time window. The birth of each body plan might be even in a much shorter time interval. It, therefore, raises a question, “Does the Cambrian Explosion pose a challenge to evolution?” The answer is straightforward. Millions or tens of millions of years is geologically short, but it is plenty of time for evolutionary change as fossil record tells us. Early animals had plenty of time to invade new niches, invent new organs, make shells, and change body design.
What is the genetic basis of the Cambrian Explosion?
Whole-genomic sequences of a broad array of metazoans have demonstrated that only a small fraction of genomes constitute developmental Gene Regulatory Networks (GRNs) that control the construction of body plans and hence is usually considered as an intrinsic prerequisite for the early diversification of metazoans. The key question is, when did GRNs that are complex enough for constructing metazoan body plans as complex as Cambrian animals evolved? Although the genetic makeup of Cambrian animals was unavailable, it can be inferred by comparative molecular developmental studies. Thus, tracing the evolution of GRNs across a robust phylogeny of the Metazoa is crucially important for understanding the diversification of metazoan body plans during the Cambrian Explosion.
The basic framework of metazoan phylogeny has been stabilized for more than 20 years (Fig. 3). Choanoflagellates have long been recognized as the closest living relatives of animals and they together constitute the Choanozoa, which, together with Filasterea and Ichthyosporea, form the Holozoa. The monophyly of the Metazoa is currently well recognized, consisting of a number of paraphyletic non-bilateral clades and the monophyletic Bilateria. The Bilateria is composed of three superphylum-level clades (deuterostomes, ecdysozoans and lophotrochozoans) and the Xenoaceolomorpha (a small group of bilaterians lacking a coelom). But some relationships within animals remain controversial. Among non-bilateral clades, ctenophores were controversially placed to be basal to the Porifera, sister to the Eumetazoan (Laumer et al. 2019), or together with the Cnidaria, revive the Coelenterata (Pett et al. 2019); the Placazoa was placed as a sister clade to the Eumetazoa (which together comprise the Epitheliozoa) or to the Cnidaria (Laumer et al. 2019). Within the Bilateria, the Xenoacoelomorpha was either basal to the Nephrozoa (protostomes and deuterostomes) or at a basal position within deuterostomes (Laumer et al. 2019; Philippe et al. 2019); there are other uncertainties within the three major bilateral clades that are beyond the scope of this discussion.
The genome evolution across the tree of metazoans shows interesting points: (1) Many of the core elements of the metazoan regulatory genome have an ancient origin among the Holozoa (Erwin 2020). (2) The developmental system of metazoans appears to be precociously configured, e.g., genes used in cell-type differentiation and patterning in metazoans are already present in choanoflagellates; sponges possess large numbers of genes otherwise found only in eumetazoans; placozoans have many genes that are important in eumetazoan development and numbers of gene families associated in a broad sampling of bilaterians with the specification of neurons and myocytes, with antero-posterior patterning, and with organogenesis, although they lack nerve or muscle cells and show no indication of bilateral features; cnidarians have many genes that mediate the development of tissues and organs in triploblastic forms (Erwin and Valentine 2013). (3) There are distinctive differences between the developmental architectures of non-bilaterians and bilaterians, relatively flat regulatory hierarchies against a more hierarchical, and more interconnected GRNs (Erwin 2020). (4) Gene gains, losses and duplications dynamically evolved and have unbalanced distributions across the tree; gene duplication ratio was high at deep nodes leading to metazoans and the birth rate decreased progressively as animals diversified into clades; around one-third of genes gained at deep nodes below the Nephrozoa and another one-third comprising linage-specific genes (no homologues in other taxa) acquired very late; it is not only key innovations but also the evolutionary dynamics of gene gain and loss that shape the vast morphological disparity of animals, notably deuterostomes, ecdysozoans, and Xenacoelomorpha were characterized by no gene gain but rampant differential gene loss (Fernández and Gabaldón 2020).
The trace-fossil record indicates bilaterians were in place by 560 Ma. The Cambrian Explosion was from above this node and non-bilateral clades must originate earlier though they were underrepresented in Precambrian deposits. The rapid diversification of pre-bilateral phyla along with bilateral clades as seen in the fossil record may have resulted from a later acquisition of linage-specific genes during the time window of ~ 560–520 Ma. Another crucial point is the complexity of the last common ancestor of protostomes and deuterostomes (PDA). Early comparative studies between vertebrates and arthropods suggested a morphologically and genetically complex PDA, with a central nervous system, gut, eyes, segmentation, and other features (e.g., Carroll et al. 2001). If so, the bilateral clades diverged without requiring the invention of novel genes. Therefore, the Cambrian Explosion was regarded as an ecological phenomenon (Carroll 2005). However, Erwin (2020) argued that deep homologies of developmental tools had limited morphological expression and thus suggested a less complex PDA either developmentally or morphologically, with a variety of cell types and patterning systems (anterior–posterior, dorsal–ventral). He also proposed that the developmental machinery for appendages, eyes, gut formation, segmentation and other features arose independently in the major bilaterian clades after the PDA, largely through extensive co-option of existing regulatory components (Erwin 2020).
Did environmental changes drive the Cambrian Explosion?
The final assemblage of the supper continent Gondwana and the opening of the Iapetus Ocean were characteristic tectonic events during Ediacaran–Cambrian transition and hence were frequently discussed as a tectonic backdrop for the Cambrian Explosion (e.g., Zhang et al. 2014). Continental-scale transgressive sequences dis- or un-conformably overlie the Precambrian rocks of varying age (Peters and Gaines 2012) and are usually rich in phosphorus. Climate might be warm or hot (Boucot et al. 2009; Hearing et al. 2018; Scotese et al. 2021). Atmosphere was well oxygenated, and the free oxygen content was constrained between 10%PAL and 40%PAL (Sperling et al. 2015; Zhang and Cui 2016). The ocean was rich in nutrient (Sperling and Stockey 2018) but not much saltier than today (Knauth 1998; Hay et al. 2006). Fluctuations of seawater chemistry, e.g., the increase of Ca2+ concentration (Arp et al. 2001; Lowenstein et al. 2003) did not obstruct the pace of evolution. Marine redox was heterogeneous in space and volatile in tempo, with anoxic or euxinic water bodies present from time to time and places to places (Wood and Erwin 2017; He et al. 2019; Wei et al. 2021). Oxygenated seafloor was expanding through time (Li et al. 2017).
Indeed, the Earth became well habitable for metazoans! On the basis of the time-equivalence, these environmental changes were often considered as extrinsic triggers of the Cambrian Explosion (see a review in Zhang et al. 2014). In particular, marine redox fluctuations were frequently proposed to be a likely trigger for the early animal innovation (e.g., Wei et al. 2021). However, these environmental changes were not unique in the time window of the Cambrian Explosion, but have all occurred during the Phanerozoic. Organisms are well known to passively response to environmental changes, but they may actively shape their environments through ecological feedbacks. Such ecological interactions are ongoing all the time, yet no explosion of body plans has been seen in the subsequent Phanerozoic. Therefore, searching for an environmental cause might be unavailing as James W. Valentine stated “Paleoenvironmental signals that can be related to the origins of body plans seem extremely different to discover. The signals from the late Neoproterozoic and Cambrian rocks are difficult to interpret, and none of them as yet confirm that any extraordinary environmental events were causally associated with the Cambrian Explosion (Valentine 2004: 519)”. Moreover, habitable conditions, notably availability of oxygen (Nursall 1959), are merely a prerequisite for diversification of body plans but they are insufficient to explain the extent of morphological innovations of the Cambrian Explosion (Erwin 2015).
Is the Cambrian Explosion an ecological phenomenon?
Both environmental habitability and developmental system were necessary parts of any explanation, but neither is a sufficient prerequisite for the Cambrian Explosion. Thus, it is becoming increasingly appreciated that the Cambrian Explosion was an ecological phenomenon (Carroll 2005; Conway Morris 2006; Budd 2008; Erwin et al. 2011; Erwin and Tweedt 2012), consisting largely of a cascade of knock-on events that emerged from multicellularity and mobility (e.g., Budd 2008). Any evolutionary event must run through ecology and the Cambrian Explosion is no exception. Metazoans have become ecologically non-negligible since the late Ediacaran. The complex ecologies were subject to both continuing expansion and feedback, thereby resulting in the evolution of metazoan-dominated ecosystem since the early Cambrian (Conway Morris 2006). Many ecological hypotheses have been proposed as drivers (see a review in Zhang et al. 2014), but none can satisfy geological, paleontological, and molecular records (Marshall 2006). The crux of these ecological drivers is new opportunities (available in new adaptive zones or released by mass extinct) and arm race. Again, ecological drivers are not unique during the Cambrian Explosion and thus fail to explain why the “explosion” happened when it did (Marshall 2006). However, the Cambrian Explosion did take place under these circumstances. Ecology must contribute the ecological expanding of metazoans and the increase of ecosystem complexity, in particular through positive feedback that is distinctive from negative feedback seen in subsequent radiation events (Erwin and Valentine 2013). However, this argument might immediately collapse when asking the following question.
Why did no new phyla arise in subsequent evolutionary radiations?
It is hard to believe that external ecology will explain the entire phenomenon of the Cambrian Explosion (Gould 1989). The main defense for this argument relies on the fact that few phyla has arisen since the closure of the Cambrian Explosion though ecological opportunities were released by “big five” mass extinctions and available during the terrestrialization in later geological periods. It sounds like a strong defense. The uniqueness of the Cambrian Explosion lies in that it was the first time for animals to explore a barrel world. Animals had attempted morphological and ecological possibilities in a short time window and generated numbers of body plans including many forms that are difficulty to be placed in high-rank taxa (phyla and classes) of the Linnaean classification system but in most cases can be assigned to stem or crown clades of current phylogenetic framework. However, not all is possible, successful options are limited, and convergence may lead to the same evolutionary solution (Conway Morris 2003). Non-functional attempts might be eliminated immediately, short-lived or temporarily successful attempts (stem groups) went extinct for restricted supply of resources or catastrophes, and only limited number of attempts survive to today with their extant descendants being classified into phyla and classes based on their distinctiveness. Most options, if not all, had already been attempted in the early history. Subsequent extinctions did not eliminate all high-rank clades. Many clades with survivors radiated after extinction but extinct forms (e.g., trilobite and chancelloriids) never occurred again! Animals did explore additional options in later radiation events (Deline et al. 2018) but new forms (e.g., terrestrial counterparts of arthropods and vertebrates) were still in the broad frame of early attempts.
How did the Cambrian Explosion happen?
The Cambrian Explosion is not purely a biotic process but a polythetic event of natural history that requires many sets of necessary conditions from molecules to forms, from isotopes to habitability, and so on. In other words, whichever was unavailable it could not happen and, reversely, when everything was ready it did not necessarily happen though it may take place at any time. The Cambrian Explosion is far more complicated than any historical event, which is extremely difficult to explain because complex patterns of causality, importance of contingency, and interactions of many different processes are all interwoven together. Complex histories do not have single, easily testable causes (Erwin and Valentine 2013). While the Cambrian Explosion did take place under circumstances when the world oceans became habitable for various forms of animals, the developmental GRNs were sufficiently complex for constructing complex forms, and resource supply was less restricted. It seems that opportunities were in every corner! Metazoans emerged earlier, and became ecologically non-negligible in the late Ediacaran, and finally dominated marine ecosystems by the closure of the Cambrian Explosion. Early metazoans shared seafloors with Ediacarans (vendobionts and tubular forms of uncertain affinity) for the last 20 million years of the Ediacaran, although their ecological relationships are less known. Metazoans might be ecologically humble during the Ediacaran Period, but they followed the path of evolving organs and systems, developing orderly repetition of body parts (e.g., segmentation), and attempting other possibilities, which enable the evolution of morphological, physiological, ecological variations and complexity. While Ediacarans kept their less differentiated body designs, tissue-grade organization, and probably osmotic physiology. Consequently, Ediacarans died off at the end of their era for unknown reasons (Darroch 2021). Thereafter metazoans rapidly diversified and generated number of phylum-rank stem or crown lineages with different fates in the first 20 million years of the Cambrian Period. Some immediately diverged in ecology and morphology and persisted to today (e.g., arthropods and mollusks), some may temporarily succeed in diversity but became extinct sooner or later, and some have remained lonely though they have survived into today.
References
Antcliffe, J.B. 2013. Questioning the evidence of organic compounds called sponge biomarkers. Palaeontology 56: 917–925.
Antcliffe, J.B., R.H.T. Callow, and M.D. Brasier. 2014. Giving the early fossil record of sponges a squeeze. Biological Reviews 89: 972–1004.
Arp, G., A. Reimer, and J. Reitner. 2001. Photosynthesis-induced biofilm calcification and calcium concentrations in Phanerozoic oceans. Science 292: 1071–1074.
Bambach, R.K., A.M. Bush, and D.H. Erwin. 2007. Autecology and the filling of ecospace: key metazoan radiations. Palaeontology 50: 1–22.
Bate, R.H., and B.A. East. 1972. The structure of the ostracode carapace. Lethaia 5: 177–194.
Bengtson, S. 1983. The early history of the Conodonta. Fossils and Strata 15: 5–9.
Bengtson, S., and Z. Yue. 1997. Fossilized metazoan embryos from the earliest Cambrian. Science 277: 1645–1648.
Bengtson, S., S. Conway Morris, B.J. Cooper, P.A. Jell, and B.N. Runnegar. 1990. Early Cambrian fossils from South Australia. Memoirs of the Association of Australasian Palaeontologists 9: 1–364.
Bentov, S., P. Zaslansky, A. Al-Sawalmih, A. Masic, P. Fratzl, A. Sagi, A. Berman, and B. Aichmayer. 2012. Enamel-like apatite crown covering amorphous mineral in a crayfish mandible. Nature Communications 3: 839.
Bentov, S., S. Abehsera, and A. Sagi. 2016a. The mineralized exoskeletons of crustaceans. In Extracellular composite matrices in arthropods, eds. E. Cohen and B. Moussian, 137–163. Cham: Springer.
Bentov, S., E.D. Aflalo, J. Tynyakov, L. Glazer, and A. Sagi. 2016b. Calcium phosphate mineralization is widely applied in crustacean mandibles. Scientific Reports 6: 22118.
Boag, T.H., S.A.F. Darroch, and M. Laflamme. 2016. Ediacaran distributions in space and time: testing assemblage concepts of earliest macroscopic body fossils. Paleobiology 42: 574–594.
Bobrovskiy, I., J.M. Hope, A.Y. Ivantsov, B.J. Nettersheim, C. Hallmann, and J.J. Brocks. 2018. Ancient steroids establish the Ediacaran fossil Dickinsonia as one of the earliest animals. Science 361: 1246–1249.
Bobrovskiy, I., J.M. Hope, B.J. Nettersheim, J.K. Volkman, C. Hallmann, and J.J. Brocks. 2020. Algal origin of sponge sterane biomarkers negates the oldest evidence for animals in the rock record. Nature Ecology & Evolution 5: 165–168.
Boëchat, I.G., A. Krüger, and R. Adrian. 2007. Sterol composition of freshwater algivorous ciliates does not resemble dietary composition. Microbial Ecology 53: 74–81.
Botting, J.P., and L.A. Muir. 2018. Early sponge evolution: a review and phylogenetic framework. Palaeoworld 27: 1–29.
Botting, J.P., and B.J. Nettersheim. 2018. Searching for sponge origins. Nature Ecology & Evolution 2: 1685–1686.
Bottjer, D.J., Z.-J. Yin, F.-C. Zhao, and M.-Y. Zhu. 2020. Comparative taphonomy and phylogenetic signal of phosphatized Weng’an and Kuanchuanpu biotas. Precambrian Research 349: 105408.
Boucot, A.J., X. Chen, C.R. Scotese, and J.-X. Fan. 2009. Reconstruction of Phanerozoic climate. Beijing: Science Press.
Brasier, M.D. 1979. The Cambrian radiation event. In The origin of major invertebrate groups, ed. M.R. House, Systematics Association Special Volume 12: 103–159. New York: Academic Press.
Briggs, D.E.G. 2015. The Cambrian explosion. Current Biology 25: R845–R875.
Briggs, D.E.G., and R.A. Fortey. 1982. The cuticle of aglaspidid arthropods, a red herring in the early history of vertebrates. Lethaia 15: 25–29.
Briggs, D.E.G., and R.E. Summons. 2014. Ancient biomolecules: their origins, fossilization, and role in revealing the history of life. BioEssays 36: 482–490.
Buatois, L.A., and M.G. Mángano. 2018. The other biodiversity record: innovations in animal-substrate interactions through geologic time. GSA Today 28: 4–10.
Buatois, L.A., G.M. Narbonne, M.G. Mángano, N.B. Carmona, and P. Myrow. 2014. Ediacaran matground ecology persisted into the earliest Cambrian. Nature Communications 5: 3544.
Budd, G.E. 2008. The earliest fossil record of the animals and its significance. Philosophical Transactions of the Royal Society (B: Biological Sciences) 363: 1425–1434.
Budd, G.E., and S. Jensen. 2000. A critical reappraisal of the fossil record of the bilaterian phyla. Biological Reviews 75: 253–295.
Budd, G.E., and R.P. Mann. 2020. Survival and selection biases in early animal evolution and a source of systematic overestimation in molecular clocks. Interface Focus 10: 20190110.
Bush, A.M., R.K. Bambach, and D.H. Erwin. 2011. Ecospace utilization during the Ediacaran radiation and the Cambrian eco-explosion. In Quantifying the evolution of early life, eds. J.D. Shiffbauer, and S.Q. Dornbos, 111–113. Dordrecht: Springer.
Cai, Y.-P., H. Hua, and X.-L. Zhang. 2013. Tube construction and life mode of the late Ediacaran tubular fossil Gaojiashania cyclus from the Gaojiashan Lagerstätte. Precambrian Research 224: 255–267.
Cai, Y.-P., S.-H. Xiao, G.-X. Li, and H. Hua. 2019. Diverse biomineralizing animals in the terminal Ediacaran period herald the Cambrian explosion. Geology 47: 380–384.
Carroll, S.B. 2005. Endless forms most beautiful: the new science of Evo Devo and the making of the animal Kingdom. New York: W.W Norton & Company.
Carroll, S.B., J.K. Grenier, and S.D. Weatherbee. 2001. From DNA to diversity. Malden, MA: Blackwell Science.
Carter, J.G., and R.M. Hall. 1990. Polyplacophora, Scaphopoda, Archaeogastropoda and Paragastropoda (Mollusca). Skeletal biomineralisation: patterns, processes and evolutionary trends, short course in geology, vol. 5(2), 297–411. Washington DC: American Geophysical Union.
Chen, J.-Y., and C. Teichert. 1983. Cambrian cephalopods. Geology 11: 647–650.
Chen, J.-Y., D.-Y. Huang, Q.-Q. Peng, H.-M. Chi, X.-Q. Wang, and M. Feng. 2003. The first tunicate from the early Cambrian of South China. Proceedings of the National Academy of Sciences of the United States of America 100: 8314–8318.
Chen, Z., S. Bengtson, C.-M. Zhou, H. Hua, and Z. Yue. 2007. Tube structure and original composition of Sinotubulites: shelly fossils from the late Neoproterozoic in southern Shaanxi, China. Lethaia 41: 37–44.
Chen, Z., X. Chen, C.-M. Zhou, X.-L. Yuan, and S.-H. Xiao. 2018. Late Ediacaran trackways produced by bilaterian animals with paired appendages. Science Advance 4: eaao6691.
Chen, Z., C.-M. Zhou, X.-L. Yuan, and S.-H. Xiao. 2019. Death march of a segemnted and trilobate bilaterian elucidates early animal evolution. Nature 573: 412–415.
Cloud, P.E. 1948. Some problems and patterns of evolution exemplified by fossil invertebrates. Evolution 2: 322–350.
Conway Morris, S. 1998. The crucible of creation—the Burgess Shale and the rise of animals. Oxford: Oxford University Press.
Conway Morris, S. 2000. The Cambrian ‘“explosion”’: slow-fuse or megatonnage? Proceedings of the National Academy of Sciences of the United States of America 97: 4426–4429.
Conway Morris, S. 2003. Life’s solution: Inevitable humans in a lonely Universe. Cambridge: Cambridge University Press.
Conway Morris, S. 2006. Darwin’s dilemma: the realities of the Cambrian ‘explosion.’ Philosophical Transactions of the Royal Society (B: Biological Sciences) 361: 1069–1083.
Conway Morris, S., and M. Chen. 1991. Cambroclaves and paracarinachitids, early skeletal problematics from the Lower Cambrian of South China. Palaeontology 34: 357–397.
Conway Morris, S., and J.S. Peel. 1990. Articulated halkieriids from the Lower Cambrian of north Greenland. Nature 345: 802–805.
Conway Morris, S., J.S. Crampton, B. Xiao, and A.J. Chapman. 1997. Lower Cambrian cambroclaves (incertae sedis) from Xinjiang, China, with comments on the morphological variability of sclerites. Palaeontology 40: 167–189.
Currey, D.J., A. Nash, and W. Bonfield. 1982. Calcified cuticle in the stomatopod smashing limb. Journal of Materials Science 17: 1939–1944.
Daley, A.C., J.B. Antcliffe, H.B. Drage, and S. Pates. 2018. Early fossil record of Euarthropoda and the Cambrian Explosion. Proceedings of the National Academy of Sciences of the United States of America 115: 5323–5331.
Darroch, S.A.F., et al. 2021. The trace fossil record of the Nama Group, Namibia: exploring the terminal Ediacaran roots of the Cambrian explosion. Earth Science Reviews 212: 103435.
Darwin, C.R. 1859. On the origin of species by means of natural selection, 1st ed. London: John Murray.
Darwin, C.R. 1872. The origin of species by means of natural selection, or the preservation of favoured races in the struggle for life, 6th ed. London: John Murray.
Degens, E.T. 1976. Molecular mechanisms on carbonate, phosphate, and silica deposition in the living cell. In Topics in Current Chemistry, eds. E.T. Degens, W.A.P. Luck, and D.D. Perrin, 1–112. Berlin: Springer.
Degens, E.T., J. Kazmierczak, and V. Ittekkot. 1985. Cellular response to Ca2+ stress and geological implications. Acta Palaeontologica Polonica 30: 115–135.
Degens, E.T., J. Kazmierczak, and V. Ittekkot. 1986. Biomineralization and the carbon isotope record. Tschermaks Mineralogische und Petrographische Mitteilungen 35: 117–126.
Deline, B., J.M. Greenwood, J.W. Clark, M.N. Puttick, K.J. Peterson, and P.C.J. Donoghue. 2018. Evolution of metazoan morphological disparity. Proceedings of the National Academy of Sciences of the United States of America 115: E8909–E8918.
Dominguez, P., A.G. Jacobson, and R.P.S. Jefferies. 2002. Paired gill slits in a fossil with a calcite skeleton. Nature 417: 841–844.
Droser, M.L., L.G. Tarhanand, and J.G. Gehling. 2017. The rise of animals in a changing environment: global ecological innovation in the late Ediacaran. Annual Review of Earth and Planetary Sciences 45: 593–617.
Duda, J.-P., M.J. van Kranendonk, V. Thiel, D. Ionescu, H. Strauss, N. Schäfer, and J. Reitner. 2016. A rare glimpse of Paleoarchean life: geobiology of an exceptionally preserved microbial mat facies from the 3.4 Ga strelley pool formation, Western Australia. PLoS ONE 11: e0147629.
Dunne, J.A., R.J. Williams, N.D. Martines, R.A. Wood, and D.H. Erwin. 2008. Compilation and network analysis of Cambrian food webs. PLoS Biology 6: e102.
Elicki, O. 2003. Als das Leben “explodierte” und eine völlig neue Welt entstand: das Kambrium. Biologie in Unserer Zeit 33: 381–389.
Erwin, D.H. 1991. Metazoan phylogeny and the Cambrian radiation. Trends in Ecology and Evolution 6: 131–134.
Erwin, D.H. 2007. Disparity: morphological pattern and developmental context. Palaeontology 50: 57–73.
Erwin, D.H. 2015. A public goods approach to major evolutionary innovations. Geobiology 13: 1–8.
Erwin, D.H. 2020. The origin of animal body plans: a view from fossil evidence and the regulatory genome. Development 147: dev182899.
Erwin, D.H., and S.M. Tweedt. 2012. Ecological drivers of the Ediacaran-Cambrian diversification of Metazoa. Evolutionary Ecology 26: 417–433.
Erwin, D.H., and J.W. Valentine. 2013. The Cambrian explosion, the construction of animal biodiversity. Greenwood Village, Colorado: Roberts and Company.
Erwin, D.H., J.W. Valentine, and J.J. Sepkoski. 1987. A comparative-study of diversification events—the early Paleozoic versus the Mesozoic. Evolution 41: 1177–1186.
Erwin, D.H., M. Laflamme, S.M. Tweedt, E.A. Sperling, D. Pisani, and K.J. Peterson. 2011. The Cambrian conundrum: early divergence and later ecological success in the early history of animals. Science 334: 1901–1907.
Fedonkin, M.A., and B.M. Waggoner. 1997. The late Precambrian fossil Kimberella is a mollusk-like bilaterian organism. Nature 388: 868–871.
Fernández, R., and T. Gabaldón. 2020. Gene gain and loss across the metazoan tree of life. Nature Ecology & Evolution 4: 524–533.
Fortey, R.A., D.E.G. Briggs, and M.A. Wills. 1996. The Cambrian evolutionary ‘explosion’: decoupling cladogenesis from morphological disparity. Biological Journal of the Linnean Society 57: 13–33.
Fu, D.-J., G.-H. Tong, T. Dai, W. Liu, Y.-N. Yang, Y. Zhang, L.-H. Cui, L.-Y. Li, H. Yun, Y. Wu, A. Sun, C. Liu, W.-R. Pei, R.R. Gaines, and X.-L. Zhang. 2019. The Qingjiang biota—a Burgess Shale-type fossil Lagerstätte from the early Cambrian of South China. Science 363: 1338–1342.
Geyer, G. 1998. Die kambrische Explosion. Paläontologische Zeitschrift 72: 7–30.
Glaessner, N.F. 1984. The dawn of animal life. A biohistorical study. Cambridge: Cambridge University Press.
Gould, S.J. 1989. Wonderful Life: the Burgess Shale and the nature of history. New York: W.W. Norton & Company.
Govindan, M., J.D. Hodge, K.A. Brown, and M. Nuñez-Smith. 1993. Distribution of cholesterol in Caribbean marine algae. Steroids 58: 178–180.
Grotzinger, J.P., W.A. Watters, and A.H. Knoll. 2000. Calcified metazoans in thrombolite-stromatolite reefs of the terminal Proterozoic Nama Group, Namibia. Paleobiology 26: 334–359.
Guensburg, T.E., and J. Sprinkle. 2001. Ecologic radiation of Cambro-Ordovician echinoderms. In The ecology of the Cambrian radiation, eds. A.Y. Zhuravlev and R. Riding, 428–444. New York: Columbia University Press.
Han, J., J.-N. Liu, Z.-F. Zhang, X.-L. Zhang, and D.-G. Shu. 2007. Trunk ornament on the palaeoscolecid worms Cricocosmia and Tabelliscolex from the Early Cambrian Chengjiang deposits of China. Acta Palaeontologica Polonica 52: 423–431.
Han, J., S. Conway Morris, D.-G. Shu, and H. Huang. 2017. Meiofaunal deuterostomes from the basal Cambrian of Shaanxi (China). Nature 542: 228–231.
Hay, W.W., A. Migdisov, A.N. Balukhovsky, C.N. Wold, S. Flögel, and E. Söding. 2006. Evaporites and the salinity of the ocean during the Phanerozoic: Implications for climate, ocean circulation and life. Palaeogeography, Palaeoclimatology, Palaeoecology 240: 3–46.
He, T.-C., M.-Y. Zhu, B.J.W. Mills, P.M. Wynn, A.Y. Zhuravlev, R. Tostevin, P.A.E. Pogge von Strandmann, A.-H. Yang, S.W. Poulton, and G.A. Shields. 2019. Possible links between extreme oxygen perturbations and the Cambrian radiation of animals. Nature Geoscience 12: 468–474.
Hearing, T.W., T.H.P. Harvey, M. Williams, M.J. Leng, A.L. Lamb, P.R. Wilby, S.E. Gabbott, A. Pohl, and Y. Donnadieu. 2018. An early Cambrian greenhouse climate. Science Advances 4: eaar5690.
Hoare, R.D., and J.J. Pojeta. 2006. Ordovician Polyplacophora (mollusca) from North America. Journal of Paleontology 80: 1–27.
Hou, X.-G., David J. Siveter, Derek J. Siveter, R.J. Aldridge, P.-Y. Cong, S.E. Gabbott, X.-Y. Ma, M.A. Purnell, and M. Williams. 2017. The Cambrian fossils of the Chengjiang, China: the flowering of early animal life, 2nd ed. Oxford: Wiley Blackwell.
Hua, H., Y.-P. Cai, X. Min, S. Chai, Q.-K. Dai, and Z.-H. Cui. 2020. Ecological diversity in terminal Ediacaran Gaojiashan biota. Earth Science Frontiers 27: 28–46.
Hughes, M., S. Gerber, and M.A. Wills. 2013. Clades reach highest morphologic disparity early in their evolution. Proceedings of the National Academy of Sciences of the United States of America 110: 13875–13879.
Jackson, D.J., L. Macis, J. Reitner, B.M. Degnan, G. Worheide, and G. Woerheide. 2007. Sponge paleogenomics reveals an ancient role for carbonic anhydrase in skeletogenesis. Science 316: 1893–1895.
Jackson, D.J., L. Macis, J. Reitner, and G. Wörheide. 2011. A horizontal gene transfer supported the evolution of an early metazoan biomineralization. BMC Evolutionary Biology 11: 238.
Jensen, S. 2003. The Proterozoic and earliest Cambrian trace fossil record: patterns, problems and perspectives. Integrative and Comparative Biology 43: 219–228.
Jensen, S., M.L. Droser, and J.G. Gehling. 2005. Trace fossil preservation and the early evolution of animals. Palaeogeography, Palaeoclimatology, Palaeoecology 220: 19–29.
Kazmierczak, J., V. Ittekkot, and E.T. Degens. 1985. Biocalcification through time: environmental challenge and cellular response. Paläontologische Zeitschrift 59: 15–33.
Khomentovsky, V.V., and G.A. Karlova. 2005. The Tommotian stage base as the Cambrian lower boundary in Siberia. Stratigraphy and Geological Correlation 13: 26–40.
Knauth, L.P. 1998. Salinity history of the earth’s early ocean. Nature 359: 554–555.
Kodner, R.B., R.E. Summons, A. Pearson, N. King, and A.H. Knoll. 2008. Sterols in a unicellular relative of the metazoans. Proceedings of the National Academy of Sciences of the United States of America 105: 9897–9902.
Kouchinsky, A.V. 2000. Skeletal microstructure of hyoliths from the early Cambrian of Siberia. Alcheringa 24: 65–81.
Kouchinsky, A.V. 2001. Mollusks, hyoliths, stenothecoids, and coeloscleritophorans. In The ecology of the Cambrian radiation, eds. A.Y. Zhuravlev and R. Riding, 326–349. New York: Columbia University Press.
Kouchinsky, A.V., and S. Bengtson. 2002. The tubewall of Cambrian anabaritids. Acta Palaeontologica Polonica 47: 431–444.
Kouchinsky, A.V., S. Bengtson, B. Runnegar, C. Skovsted, M. Steiner, and M. Vendrasco. 2012. Chronology of early Cambrian biomineralization. Geological Magazine 149: 221–251.
Kruse, P.D., A.Y. Zhuravlev, and N.P. James. 1995. Primordial metazoan-calcimicrobial reefs: Tommotian (Early Cambrian) of the Siberian Platform. Palaios 10: 291–321.
Kupriyanova, E.K., T.A. Mcdonald, and G.W. Rouse. 2010. Phylogenetic relationships within Serpulidae (Sabellida, Annelida) inferred from molecular and morphological data. Zoologica Scripta 35: 421–439.
Landing, E., and B. Kröger. 2009. The oldest cephalopods from East Laurentia. Journal of Paleontology 83: 123–127.
Landing, E., A. English, and J.D. Keppie. 2010. Cambrian origin of all skeletalized metazoan phyla—discovery of earth’s oldest bryozoans (Upper Cambrian, southern Mexico). Geology 38: 547–550.
Laumer, C.E., R. Fernandez, S. Lemer, D. Combosch, K.M. Kocot, A. Riesgo, S.C.S. Andrade, W. Sterrer, M.V. Sørensen, and G. Giribet. 2019. Revisiting metazoan phylogeny with genomic sampling of all phyla. Proceedings of the Royal Society (Biological Sciences) 286: 20190831.
Lefebvre, B. 2007. Early Palaeozoic palaeobiogeography and palaeoecology of stylophoran echinoderms. Palaeogeography, Palaeoclimatology, Palaeoecology 245: 156–199.
Lepot, K. 2020. Signatures of early microbial life from the Archean (4 to 2.5 Ga) eon. Earth Science Reviews 209: 103296.
Levinton, J.S. 1992. The big bang of animal evolution. Scientific American 267: 84.
Levinton, J.S. 2001. Genetics, paleontology, and macroevolution, 2nd ed. Cambridge: Cambridge University Press.
Li, G.-X., M. Steiner, X.-J. Zhu, A.-H. Yang, H.-F. Wang, and B.D. Erdtmann. 2007. Early Cambrian metazoan fossil record of South China: generic diversity and radiation patterns. Palaeogeography, Palaeoclimatology, Palaeoecology 254: 229–249.
Li, C., C.-S. Jin, N.J. Planavsky, T.J. Algeo, M. Cheng, X.-L. Yang, Y.-L. Zhao, and S.-C. Xie. 2017. Coupled oceanic oxygenation and metazoan diversification during the early–middle Cambrian? Geology 45: 743–746.
Lin, J.P., A.Y. Ivantsov, and D.E.G. Briggs. 2010. The cuticle of the enigmatic arthropod Phytophilaspis and biomineralization in Cambrian arthropods. Lethaia 44: 344–349.
Liu, Y., X.-L. Zhang, W. Liu, and Q. Zhang. 2008. New bradoriids from the lower Cambrian Yanwangbian formation of southern Shaanxi Province, Central China. Palaeoworld 17: 102–107.
Liu, A.G., J.J. Matthews, L.R. Menon, D. McIlroy, and M.D. Brasier. 2014a. Haootia quadriformis n. gen., n. sp., interpreted as a muscular cnidarian impression from the late Ediacaran Period (approx. 560 Ma). Proceedings of the Royal Society (Biological Sciences) 281: 20141202.
Liu, Y.-H., S.-H. Xiao, T.Q. Shao, J. Broce, and H.-Q. Zhang. 2014b. The oldest known priapulid-like scalidophoran animal and its implications for the early evolution of cycloneuralians and ecdysozoans. Evolution & Development 16: 155–165.
Love, G.D., and J.A. Zumberge. 2021. Emerging patterns in Proterozoic lipid biomarker records: Implications for marine biospheric evolution and the ecological rise of eukaryotes. In Elements in geochemical tracers in earth system science, eds. T. Lyons, A. Turchyn, and C. Reinhard, 1–75. Cambridge: Cambridge University Press.
Love, G.D., E. Grosjean, C. Stalvies, D.A. Fike, J.P. Grotzinger, A.S. Bradly, A.E. Kelly, M. Bhatia, W. Meredith, C.E. Snape, S.A. Bowring, D.J. Condon, and R.E. Summons. 2009. Fossil steroids record the appearance of Demospongiae during the Cryogenian period. Nature 457: 718–721.
Lowenstam, H.A., and D. Abbott. 1975. Vaterite: a mineralization product of the hard tissues of a marine organism (Ascidiacea). Science 188: 363–365.
Lowenstam, H.A., and S. Weiner. 1992. Phosphatic shell plate of the barnacle Ibla (Cirripedia): a bone-like structure. Proceedings of the National Academy of Sciences of the United States of America 89: 10573–10577.
Lowenstein, T.K., M.N. Timofeeff, S.T. Brennan, L.A. Hardie, and R.V. Demicco. 2001. Oscillations in Phanerozoic seawater chemistry: evidence from fluid inclusions. Science 294: 1086–1088.
Lowenstein, T.K., L.A. Hardie, M.N. Timofeeff, and R.V. Demicco. 2003. Secular variation in seawater chemistry and the origin of calcium chloride basinal brines. Geology 31: 857–860.
Lozano-Fernandez, J.R., A.R. Carton, M.N. Tanner, M. Puttick, J. Blaxter, J. Vinther, G. Olesen, G.D. Edgecombe. Giribet, and D. Pisani. 2016. A molecular palaeobiological exploration of arthropod terrestrialization. Philosophical Transaction of the Royal Society (B: Biological Sciences) 371: 20150133.
Maletz, J. 2019. Tracing the evolutionary origins of the Hemichordata (Enteropneusta and Pterobranchia). Palaeoworld 28: 58–72.
Maldegem, L.M. van, B.J. Nettersheim, A. Leider, J.J. Brocks, P. Adam, P. Schaeffer, and C. Hallmann. 2020. Geological alteration of Precambrian steroids mimics early animal signatures. Nature Ecology & Evolution 5: 169–173.
Maloof, A., S.M. Porter, J.L. Moores, F.O. Dudas, S.A. Bowring, J.A. Higgins, D.A. Fike, and M.P. Eddy. 2010. The earliest Cambrian record of animals and ocean geochemical change. Geological Society of America Bulletin 122: 1731–1774.
Mángano, M.G., and L.A. Buatois. 2014. Decoupling of body-plan diversification and ecological structuring during the Ediacaran-Cambrian transition: evolutionary and geobiological feedbacks. Proceedings of the Royal Society (B: Biological Sciences) 281: 20140038.
Mángano, M.G., and L.A. Buatois. 2016. The Cambrian explosion. In The trace fossil record of major evolutionary events, eds. M.G. Mángano, and L.A. Buatois. Topics in Geobiology 39: 73–126.
Mángano, M.G., and L.A. Buatois. 2020. The rise and early evolution of animals: where do we stand from a trace-fossil perspective? Interface Focus 10: 20190103.
Marshall, C.R. 2006. Explaining the Cambrian ‘“explosion”’ of animals. Annual Review of Earth and Planetary Science 34: 355–384.
Marusin, V.V., and D.V. Grazhdankin. 2018. Enigmatic large-sized tubular fossils from the Terreneuvian of Arctic Siberia. PalZ. Paläontologische Zeitschrift 92: 557–560.
McIlroy, D., and H. Szaniawski. 2010. A lower Cambrian protoconodont apparatus from the Placentian of southeastern Newfoundland. Lethaia 33: 95–102.
Miller, J.F. 1984. Cambrian and earliest Ordovician conodont evolution, biofacies, and provincialism. Special Paper of the Geological Society of America 196: 43–68.
Mißbach, H., J.-P. Duda, A.M. van den Kerkhof, V. Lüders, A. Pack, J. Reitner, and V. Thiel. 2021. Ingredients for microbial life preserved in 3.5 billion-year-old fluid inclusions. Nature Communications 12: 1101.
Müller, K.J. 1979. Phosphatocopine ostracodes with preserved appendages from the upper Cambrian of Sweden. Lethaia 12: 1–27.
Müller, W.E.G., X.H. Wang, S.I. Belikov, and H.C. Schröder. 2008. Formation of siliceous spicules in demosponges: example suberites domuncula. In Handbook of biomineralization: biological aspects and structure formation, ed. E. Bäuerlein, 59–82. Weinheim: Wiley-VCH.
Murdock, D.J.E. 2020. The ‘biomineralization toolkit’ and the origin of animal skeletons. Biological Reviews 95: 1372–1392.
Nash, J.M. 1995. When life exploded. Time Magazine 146: 23.
Nettersheim, B.J., J.J. Brocks, A. Schwelm, and F. Not. 2019. Putative sponge biomarkers in unicellular Rhizaria question an early rise of animals. Nature Ecology & Evolution 3: 577–581.
Nursall, J. 1959. Oxygen as a prerequisite to the origin of the Metazoa. Nature 183: 1170–1172.
Parkhaev, P. 2017. Origin and the early evolution of the phylum Mollusca. Paleontological Journal 51: 91–112.
Paterson, J.R., G.D. Edgecombe, and M.S.Y. Lee. 2019. Trilobite evolutionary rates constrain the duration of the Cambrian Explosion. Proceedings of the National Academy of Sciences of the United States of America 116: 4394–4399.
Payne, J.L., A.G. Boyer, J.H. Brown, S. Finnegan, M. Kowalewski, R.A. Krause Jr., S.K. Lyons, C.R. McClain, D.W. McShea, P.M. Novack-Gottshall, F.A. Smith, J.A. Stempien, and S.-C. Wang. 2009. Two-phase increase in the maximum size of life over 3.5 billion years reflects biological innovation and environmental opportunity. Proceedings of the National Academy of Sciences of the United States of America 106: 24–27.
Pecoits, E., K.O. Konhauser, N.R. Aubet, L.M. Heaman, G. Veroslavsky, R.A. Stern, and M.K. Gingras. 2012. Bilaterian burrows and grazing behavior at >585 million years ago. Science 336: 1693–1696.
Peel, J.S. 2006. Scaphopodization in palaeozoic molluscs. Palaeontology 49: 1357–1364.
Peng, S.-C., L.E. Babcock, and R.A. Ahlberg. 2020. The Cambrian period. In Geologic time scale 2020, eds. F.M. Gradstein, J.G. Ogg, M.D. Schmitz, and G.M. Ogg, 565–629. Amsterdam: Elsevier.
Peters, S.E., and R.R. Gaines. 2012. Formation of the ‘Great Unconformity’ as a trigger for the Cambrian Explosion. Nature 484: 363–366.
Pett, W., M. Adamski, M. Adamska, W.R. Francis, M. Eitel, D. Pisani, and G. Wörheide. 2019. The role of homology and orthology in the phylogenomic analysis of metazoan gene content. Molecular Biology and Evolution 36: 643–649.
Pflug, H.D. 1972. Systematik der jung-präkambrischen Petalonamae. Paläontologische Zeitschrift 46: 56–67.
Pflug, H.D. 1974. Vor- und Frühgeschichte der Metazoa. Neues Jahrbuch für Geologie und Paläontologie, Abhandlungen 145: 328–337.
Philippe, H., A.J. Poustka, M. Chiodin, K.J. Hoff, C. Dessimoz, B. Tomiczek, P.H. Schiffer, S. Müller, D. Domman, M. Horn, et al. 2019. Mitigating anticipated effects of systematic errors supports sister-group relationship between xenacoelomorpha and ambulacraria. Current Biology 29: 1818–1826.
Pisani, D., and A.G. Liu. 2015. Animal evolution: only rocks can set the clock. Current Biology 25: R1079–R1081.
Porter, S.M. 2007. Seawater chemistry and early carbonate biomineralization. Science 316: 1302.
Reis, M. dos, Y. Thawornwattana, K. Angelis, M.J. Telford, P.C. Donoghue, and Z. Yang. 2015. Uncertainty in the timing of origin of animals and the limits of precision in molecular timescales. Current Biology 25: 2939–2950.
Reitner, J., and D. Mehl. 1995. Early Palaozoic diversification of sponges: new data and evidence. Geologisch-Paläontologische Mitteilungen Innsbruck 20: 335–347.
Reitner, J., and D. Mehl. 1996. Monophyly of the Porifera. Verhandlungen des Naturwissenschaftlichen Vereins in Hamburg 36: 5–32.
Rigby, J.K. 1986. Sponges of the Burgess Shale (middle Cambrian), British Columbia. Palaeontographica Canadiana 2: 1–105.
Rota-Stabelli, O., A.C. Daley, and D. Pisani. 2013. Molecular timetrees reveal a Cambrian colonization of land and a new scenario for ecdysozoan evolution. Current Biology 23: 392–398.
Rozanov, A.Y., and A.Y. Zhuravlev. 1992. The lower Cambrian fossil record of the Soviet Union. In Origin and early evolution of the Metazoa, eds. J.H. Lipps and P.W. Signor, 205–282. New York & London: Plenum Press.
Rücklin, M., P.C.J. Donoghue, Z. Johanson, K. Trinajstic, F. Marone, and M. Stampanoni. 2012. Development of teeth and jaws in the earliest jawed vertebrates. Nature 491: 748–751.
Runnegar, B. 1982. The Cambrian Explosion—animals or fossils. Journal of the Geological Society of Australia 29: 395–411.
Runnegar, B. 1985. Shell microstructure of Cambrian molluscs replicated by calcite. Alcheringa 9: 245–257.
Runnegar, B., and C. Bentley. 1983. Anatomy, ecology and affinities of the Australian early Cambrian bivalve Pojetaia runnegari Jell. Journal of Paleontology 57: 73–92.
Schiffbauer, J.D. 2016. The age of tubes: a window into biological transition at the Precambrian-Cambrian boundary. Geology 44: 975–976.
Schiffbauer, J.D., J.W. Huntley, G.R. O’Neil, S.A.F. Darroch, M. Laflamme, and Y. Cai. 2016. The latest Ediacaran Wormworld fauna: setting the ecological stage for the Cambrian explosion. GSA Today 26: 4–11.
Schopf, J.W. 2000. Solution to Darwin’s dilemma: discovery of the missing Precambrian record of life. Proceedings of the National Academy of Sciences of the United States of America 97: 6947–6953.
Scotese, C.R., H.-J. Song, J.W.B. Mills, and D.G. van der Meer. 2021. Phanerozoic paleotemperatures: the earth’s changing climate during the last 540 million years. Earth Science Reviews 215: 103503.
Sdzuy, K. 1960. Zur Wende Präkambrium/Kambrium. Paläontologische Zeitschrift 34: 154–160.
Seilacher, A. 1956. Der Beginn des Kambriums als biologische Wende. Neues Jahrbuch für Geologie und Paläontologische, Abhandlungen 103: 155–180.
Seilacher, A. 1989. Vendozoa: organismic construction in the Proterozoic biosphere. Lethaia 22: 229–239.
Seilacher, A. 1992. Vendobionta and Psammocorallia: lost constructions of the Precambrian evolution. Journal of the Geological Society of London 149: 607–613.
Seilacher, A. 1997. The meaning of the Cambrian explosion. In The Cambrian explosion and the fossil record, eds. J.-Y. Chen and A. Seilacher. Bulletin of National Museum of Natural Science 10: 1–9.
Seilacher, A. 1999. Biomat-related lifestyles in the Precambrian. Palaios 14: 86–93.
Seilacher, A. 2007. The nature of vendobionts. In The rise and fall of the Ediacaran biota, eds. P. Vichers-Rich and P. Komarower, 387–397. London: Geological Society.
Shen, Y.-A., R. Buick, and D.E. Canfield. 2001. Isotopic evidence for microbial sulfate reduction in the early Archaean era. Nature 410: 77–81.
Shu, D.-G. 2008. Cambrian explosion: birth of animal tree. Gondwana Research 14: 219–240.
Shu, D.-G., and J. Han. 2020. The core value of Chengjiang fauna: the formation of the animal kingdom and the birth of basic human organs. Earth Science Frontiers 27: 382–412.
Shu, D.-G., Y. Isozaki, X.-L. Zhang, J. Han, and S. Maruyama. 2014. The birth and evolution of metazoans. Gondwana Research 25: 884–895.
Simkiss, K. 1977. Biomineralization and detoxification. Calcified Tissue Research 24: 199–200.
Simpson, G.G. 1944. Tempo and mode in evolution. New York: Columbia University Press.
Skovsted, C.B. 2003. Mobergellans (Problematica) from the Cambrian of Greenland, Siberia and Kazakhstan. Paläontologische Zeitschrift 77: 429–443.
Skovsted, C.B., and L.E. Holmer. 2003. The early Cambrian (Botomian) stem group brachiopod Mickwitzia from Northeast Greenland. Acta Palaeontologica Polonica 48: 1–20.
Skovsted, C.B., and J.S. Peel. 2011. Hyolithellus in life position from the lower Cambrian of North Greenland. Journal of Paleontology 85: 37–47.
Skovsted, C.B., G.A. Brock, J.R. Paterson, L.E. Holmer, and G.E. Budd. 2008. The scleritome of Eccentrotheca from the lower Cambrian of South Australia: Lophophorate affinities and implications for tommotiid phylogeny. Geology 36: 171–174.
Smith, M.P., and D.A.T. Harper. 2013. Causes of the Cambrian explosion. Science 341: 1355–1356.
Sorauf, J.E., and M. Savarese. 1995. A lower Cambrian coral from South Australia. Palaeontology 38: 757–770.
Sperling, E.A., and R.G. Stockey. 2018. The temporal and environmental context of early animal evolution: considering all the ingredients of an “explosion.” Integrative and Comparative Biology 58: 605–622.
Sperling, E.A., and J. Vinther. 2010. A placozoan affinity for Dickinsonian and the evolution of the late Proterozoic metazoan feeding modes. Evolution and Development 12: 201–209.
Sperling, E.A., J.M. Robinson, D. Pisani, and K.J. Peterson. 2010. Where is the glass? Biomarkers, molecular clocks, and microRNAs suggest a 200-Myr missing Precambrian fossil record of siliceous sponge spicules. Geobiology 8: 24–36.
Sperling, E.A., C.J. Wolock, A.S. Morgan, B.C. Gill, M. Kunzmann, G.P. Halverson, F.A. Macdonald, A.H. Knoll, and D.T. Johnston. 2015. Statistical analysis of iron geochemical data suggests limited late Proterozoic oxygenation. Nature 523: 451–451.
Steiner, M., G.-X. Li, Y. Qian, M.-Y. Zhu, and B.D. Erdtmann. 2007. Neoproterozoic to early Cambrian small shelly fossil assemblages and a revised biostratigraphic correlation of the Yangtze Platform (China). Palaeogeography, Palaeoclimatology, Palaeoecology 254: 67–99.
Summons, R.E., and D.H. Erwin. 2018. Chemical clues to the earliest animal fossils. Science 361: 1198–1199.
Tashiro, T., A. Ishida, M. Hori, M. Igisu, M. Koike, P. Méjean, N. Takahata, Y. Sano, and T. Komiya. 2017. Early trace of life from 3.95 Ga sedimentary rocks in Labrador, Canada. Nature 549: 516–518.
Taylor, P.D., and M.J. Weedon. 2000. Skeletal ultrastructure and phylogeny of cyclostome bryozoans. Zoological Journal of the Linnean Society 128: 337–399.
Taylor, P.D., B. Berning, and M.A. Wilson. 2013. Reinterpretation of the Cambrian ‘Bryozoan’ Pywackia as an octocoral. Journal of Paleontology 87: 984–990.
Teigler, D.J., and K.M. Towe. 1975. Microstructure and composition of the trilobite exoskeleton. Fossils and Strata 4: 137–149.
Topper, T.P., and C.B. Skovsted. 2017. Keeping a lid on it: muscle scars and the mystery of the Mobergellidae. Zoological Journal of the Linnean Society 180: 717–731.
Topper, T.P., J.-F. Guo, S. Clausen, C.B. Skovsted, and Z.-F. Zhang. 2019. A stem group echinoderm from the basal Cambrian of China and the origins of Ambulacraria. Nature Communications 10: 1366.
Topper, T.P., J. Guo, S. Clausen, C.B. Skovsted, and Z.-F. Zhang. 2020. Reply to ‘Re-evaluating the phylogenetic position of the enigmatic early Cambrian deuterostome Yanjiahella’. Nature Communications 11: 1287.
Treves, K., W. Traub, S. Weiner, and L. Addadi. 2003. Aragonite formation in the chiton (Mollusca) girdle. Helvetica Chimica Acta 86: 1101–1112.
Tynan, M.C. 1983. Coral-like microfossils from the lower Cambrian of California. Journal of Paleontology 57: 1188–1211.
Ushatinskaya, G.T., and Ya..E.. Malakhovskaya. 2006. The first brachiopods with a carbonate skeleton: appearance, migration, shell wall structure. In The evolution of biosphere and biodiversity, ed. S.V. Rozhnov, 177–192. Moscow: Tovarishchestvo nauchnykh izdaniy KMK.
Ushatinskaya, G.T., and A.Y. Zhuravlev. 1994. On the problem of the skeletal biomineralisation (brachiopod example). Doklady Akademii Nauk 337: 231–234. (in Russian).
Valentine, J.W. 2004. On the origin of phyla. Chicago and London: The University of Chicago Press.
Valentine, J.W., and D.H. Erwin. 1987. Interpreting great developmental experiments: the fossil record. In Development as an evolutionary process, ed. R.A. Raff, 71–107. New York: A. R. Liss.
Vermeij, G.J. 1990. The origin of skeletons. Palaios 4: 585–589.
Vinn, O. 2006. Possible cnidarian affinities of Torellella (Hyolithelminthes, upper Cambrian, Estonia). Paläontologische Zeitschrift 80: 84–89.
Vinther, J., and C. Nielsen. 2005. The early Cambrian Halkieria is a mollusc. Zoologica Scripta 34: 81–89.
Vittori, M., V. Srot, K. Žagar, B. Bussmann, P.A. van Aken, M. Čeh, and J. Štrus. 2016. Axially aligned organic fibers and amorphous calcium phosphate form the claws of a terrestrial isopod (Crustacea). Journal of Structural Biology 195: 227–237.
Waggoner, B.M. 2003. The Ediacaran biotas in space and time. Integrative and Comparative Biology 43: 104–113.
Watson, T. 2020. The bizarre species that are rewriting animal evolution. Nature 586: 662–665.
Wei, G.-Y., N.J. Planavsky, T.-C. He, F.-F. Zhang, R.-G. Stockey, D.-B. Cole, Y.-B. Lin, and H.-F. Ling. 2021. Global marine redox evolution from the late Neoproterozoic to the early Paleozoic constrained by the integration of Mo and U isotope records. Earth-Science Reviews 214: 103506.
Weiner, S., and P.M. Dove. 2003. An overview of biomineralization processes and the problem of the vital effect. Reviews in Mineralogy and Geochemistry 54: 1–29.
Whittington, H.B. 1979. Early arthropods, their appendages and relationships. In The origin of major invertebrate groups, systematics association special volume, vol. 12, ed. M.R. House, 253–268. New York: Academic Press.
Wolfe, J.M. 2017. Metamorphosis is ancestral for crown euarthropods, and evolved in the Cambrian or earlier. Integrative and Comparative Biology 57: 499–509.
Wood, R.A. 2011. Paleoecology of the earliest skeletal metazoan communities: implications for early biomineralisation. Earth-Science Reviews 106: 184–190.
Wood, R., and D.H. Erwin. 2017. Innovation not recovery: dynamic redox promotes metazoan radiations. Biological Reviews 93: 863–873.
Wood, R., and A. Penny. 2018. Substrate growth dynamics and biomineralization of an Ediacaran encrusting poriferan. Proceedings of the Royal Society (B: Biological Sciences) 285: 20171938.
Wood, R., and A.Y. Zhuravlev. 2012. Escalation and ecological selectively of mineralogy in the Cambrian radiation of skeletons. Earth-Science Reviews 115: 249–261.
Wood, R.A., J.P. Grotzinger, and J.A.D. Dickson. 2002. Proterozoic modular biomineralized metazoan from the Nama Group, Namibia. Science 296: 2383–2386.
Wray, G.A. 2015. Molecular clocks and the early evolution of metazoan nervous systems. Philosophical Transactions of the Royal Society (Biological Sciences) 370: 20150046.
Wu, Y., D.-J. Fu, J.-X. Ma, W.-L. Lin, A. Sun, X.-L. Zhang. 2021a. Houcaris gen. nov. from the early Cambrian (Stage 3) Chengjiang Lagerstätte expanded the palaeogeographical distribution of tamisiocaridids (Panarthropoda: Radiodonta). PalZ. Paläontologische Zeitschrift. https://doi.org/10.1007/s12542-020-00545-4.
Wu, Y., J.-X. Ma, W.-L. Lin, A. Sun, X.-L. Zhang, and D.-J. Fu. 2021b. New anomalocaridids (Panarthropoda: Radiodonta) from the lower Cambrian Chengjiang Lagerstätte: biostratigraphic and paleobiogeographic implications. Palaeogeography, Palaeoclimatology, Palaeoecology 569: 110333.
Xiao, S.-H., and A.H. Knoll. 2000. Phosphatized animal embryos from the Neoproterozoic Doushantuo formation at Weng’an, Guizhou, South China. Journal of Paleontology 74: 767–788.
Xiao, S.-H., and M. Laflamme. 2009. On the eve of animal radiation: phylogeny, ecology, and evolution of the Ediacara biota. Trends in Ecology and Evolution 24: 31–40.
Yang, B., M. Steiner, M.Y. Zhu, G.X. Li, J.N. Liu, and P.J. Liu. 2016. Transitional Ediacaran-Cambrian small skeletal fossil assemblages from South China and Kazakhstan: implications for chronostratigraphy and metazoan evolution. Precambrian Research 285: 202–215.
Yang, B., M. Steiner, J.D. Schiffbauer, T. Selly, X.W. Wu, C. Zhang, and P.J. Liu. 2020. Ultrastructure of Ediacaran cloudinids suggests diverse taphonomic histories and affinities with non-biomineralized annelids. Scientific Reports 10: 535.
Yin, Z.-J., K. Vargas, J. Cunningham, S. Bengtson, M.-Y. Zhu, F. Marone, and P. Donoghue. 2019. The early Ediacaran Caveasphaera foreshadows the evolutionary origin of animal-like embryology. Current Biology 29: 4307–4314.
Yun, H., X.-L. Zhang, G.A. Brock, L.-Y. Li, and G.-X. Li. 2021. Biomineralization of the Cambrian chancelloriids. Geology. https://doi.org/10.1130/G48428.1.
Zamora, S., D.F. Wright, R. Mooi, B. Lefebvre, T.E. Guensburg, P. Gorzelak, B. David, C.D. Sumrall, S.R. Cole, A.W. Hunter, J. Sprinkle, J.R. Thompson, T.A.M. Ewin, O. Fatka, E. Nardin, M. Reich, M. Nohejlová, and I.A. Raman. 2020. Re-evaluating the phylogenetic position of the enigmatic early Cambrian deuterostome Yanjiahella. Nature Communications 11: 1286.
Zeng, H., F.-C. Zhao, K.-C. Niu, M.-Y. Zhu, and D.-Y. Huang. 2020. An early Cambrian euarthropod with radiodont-like raptorial appendages. Nature 588: 101–105.
Zhan, R.-B. 2018. Evolution of Early Paleozoic marine faunas. In Life evolution and environments, eds. J.-Y. Rong, X.-L. Yuan, R.-B. Zhan, and T. Deng, 116–143. Hefei: China University of Science and Technology. (in Chinese).
Zhang, X.-L., and L.-H. Cui. 2016. Oxygen requirements for the Cambrian explosion. Journal of Earth Science 27: 187–195.
Zhang, X.-L., and J. Reitner. 2006. A fresh look at Dickinsonian: removing it from the Vendobionta. Acta Geologica Sinica 80: 636–642.
Zhang, X.-L., and D.-G. Shu. 2014. Causes and consequences of the Cambrian explosion. Science China-Earth Sciences 57: 930–942.
Zhang, Y., and X.-L. Zhang. 2017. New Megasphaera-like microfossils reveal their reproductive strategies. Precambrian Research 300: 141–150.
Zhang, X.-L., D.-G. Shu, J. Han, Z.-F. Zhang, J.-N. Liu, and D.-J. Fu. 2014. Triggers for the Cambrian explosion: hypotheses and problems. Gondwana Research 25: 896–909.
Zhang, X.-L., P. Ahlberg, L.E. Babcock, D.K. Choi, G. Geyer, R. Gozalo, J.S. Hollingsworth, G.-X. Li, E.B. Naimark, T. Pegel, M. Steiner, T. Wotte, and Z.-F. Zhang. 2017b. Challenges in defining the base of Cambrian series 2 and stage 3. Earth-Science Reviews 172: 124–139.
Zhang, X.-L., W. Liu, Y. Isozaki, and T. Sato. 2017a. Centimeter-wide worm-like fossils from the lowest Cambrian of South China. Scientific Reports 7: 14504.
Zhu, M.-Y., A.Y. Zhuravlev, R.A. Wood, F.-C. Zhao, and S.S. Sukhov. 2017. A deep root for the Cambrian explosion: implications of new bio- and chemostratigraphy from the Siberian Platform. Geology 45: 459–462.
Zhu, M.-Y., F.-C. Zhao, Z.-J. Yin, H. Zeng, and G.-X. Li. 2019. The Cambrian explosion: advances and perspectives from China. Science China Earth Sciences 49: 1455–1490.
Zhuravlev, A.Y. 1993. Were Ediacaran Vendobionta multicellulars? Neues Jahrbuch für Geologie und Paläontologie, Abhandlungen 103: 155–180.
Zhuravlev, A.Y., and R. Wood. 2008. Eve of biomineralization: controls on skeletal mineralogy. Geology 36: 923–926.
Zhuravlev, A.Y., and R. Wood. 2020. Dynamic and synchronous changes in metazoan body size during the Cambrian Explosion. Scientific Reports 10: 6784.
Zumberge, J.A., G.D. Love, P. Cárdenas, E.A. Sperling, S. Gunasekera, M. Rohrssen, E. Grosjean, J.P. Grotzinger, and R.E. Summons. 2018. Demosponge steroid biomarker 26-methylstigmastane provides evidence for Neoproterozoic animals. Nature Ecology & Evolution 2: 1709–1714.
Acknowledgements
We are grateful to Linhao Cui, Cong Liu, and Yuhen Qiao for technical assistance and Hong Hua for providing a photograph of Figure 3. Thanks also go to Joachim Reitner (Göttingen) and Mike Reich (Munich) for constructive comments. This research was supported by Natural Science Foundation of China (41890845, 41621003, 41930139, 41772011), 111 Project (D17013), and Key Scientific and Technological Innovation Team Project in Shaanxi Province.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Joachim Reitner.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, X., Shu, D. Current understanding on the Cambrian Explosion: questions and answers. PalZ 95, 641–660 (2021). https://doi.org/10.1007/s12542-021-00568-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12542-021-00568-5