Abstract
Purpose of Review
The purpose of this review is to clarify the risks associated with MRI exams for patients with cardiac implantable electronic devices (CIEDs) and to provide information regarding the MRI examination protocol for patients with CIEDs.
Recent Findings
Several prospective studies evaluated the feasibility of MRI exams for patients with CIEDs and reported no adverse events. These studies suggest that by following a specific MRI examination protocol and monitoring both CIED parameters and the patient’s symptoms, an MRI exam can be performed by appropriately trained personnel with an acceptable benefit-to-risk ratio.
Summary
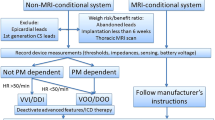
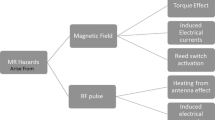
Both MR unsafe and MR conditional CIEDs are commercially available, but there are no MR safe CIEDs. The potential risks faced by patients with CIEDs during an MRI exam are always present and warrant careful monitoring. Three magnetic fields in the MRI scanner interact with the device in ways that can damage the CIED or harm the patient. Due to safety concerns and out of an abundance of caution, the majority of MRI exams for patients with CIEDs are currently denied. However, when following a specific MRI exam protocol, these risks can be mitigated.




Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Heart disease and stroke statistics—2018 update: a report from the American Heart Association 426.
Guidance for industry and FDA staff: Establishing safety and compatibility of passive implants in the magnetic resonance (MR) environment. U.S. Food and Drug Administration web site. http://www.fda.gov/cdrh. 3. Establishing safety and compatibility of passive implants in the magnetic resonance (MR) environment.
Kalin R, Stanton MS. Current clinical issues for MRI scanning of pacemaker and defibrillator patients. Pacing Clin Electrophysiol. 2005;28:326–8.
Shellock R & D Services, Inc. and Frank G. Shellock, Ph.D. (2019)
Ferreira AM, Costa F, Tralhão A, Marques H, Cardim N, Adragão P. MRI-conditional pacemakers: current perspectives. Med Devices (Auckl). 2014;7:115–24.
Pavlicek W, Geisinger M, Castle L, Borkowski GP, Meaney TF, Bream BL, et al. The effects of nuclear magnetic resonance on patients with cardiac pacemakers. Radiology. 1983;147:149–53.
• Panych LP, Madore B. The physics of MRI safety. J Magn Reson Imaging. 2018;47:28–43 A clear explanation of the interactions and risks between the mRI scanner and medical devices.
Luechinger R, Duru F, Scheidegger MB, Boesiger P, Candinas R. Force and torque effects of a 1.5-tesla MRI scanner on cardiac pacemakers and ICDs. Pacing Clin Electrophysiol. 2001;24:199–205.
Kalb B, Indik JH, Ott P, Martin DR. MRI of patients with implanted cardiac devices. J Magn Reson Imaging. 2018;47:595–603.
Tandri H, Zviman MM, Wedan SR, Lloyd T, Berger RD, Halperin H. Determinants of gradient field-induced current in a pacemaker lead system in a magnetic resonance imaging environment. Heart Rhythm. 2008;5:462–8.
Irnich W, Irnich B, Bartsch C, Stertmann W, Gufler H, Weiler G. Do we need pacemakers resistant to magnetic resonance imaging? Europace. 2005;7:353–65.
Calcagnini G, Triventi M, Censi F, Mattei E, Bartolini P, Kainz W, et al. In vitro investigation of pacemaker lead heating induced by magnetic resonance imaging: role of implant geometry. J Magn Reson Imaging. 2008;28:879–86.
Nordbeck P, Weiss I, Ehses P, Ritter O, Warmuth M, Fidler F, et al. Measuring RF-induced currents inside implants: impact of device configuration on MRI safety of cardiac pacemaker leads. Magn Reson Med. 2009;61:570–8.
Mattei E, Calcagnini G, Censi F, Triventi M, Bartolini P. Role of the lead structure in MRI-induced heating: in vitro measurements on 30 commercial pacemaker/defibrillator leads. Magn Reson Med. 2012;67:925–35.
Higgins JV, Gard JJ, Sheldon SH, et al. Safety and outcomes of magnetic resonance imaging in patients with abandoned pacemaker and defibrillator leads. Pacing Clin Electrophysiol PACE. 2014;37:1284–90.
Allison J, Yanasak N. What MRI sequences produce the highest specific absorption rate (SAR), and is there something we should be doing to reduce the SAR during standard examinations? Am J Roentgenol. 2015;205:W140.
Nordbeck P, Ritter O, Weiss I, Warmuth M, Gensler D, Burkard N, et al. Impact of imaging landmark on the risk of MRI-related heating near implanted medical devices like cardiac pacemaker leads. MRM 2011;65:44–50.
Acikel V, Magrath P, Parker SE, Hu P, Wu HH, Finn JP, et al. RF induced heating of pacemaker/ICD lead-tips during MRI scans at 1.5T and 3T: evaluation in cadavers. J Cardiovasc Magn Reson. 2016;18:O121.
Shah AD, Morris MA, Hirsh DS, Warnock M, Huang Y, Mollerus M, et al. Magnetic resonance imaging safety in nonconditional pacemaker and defibrillator recipients: a meta-analysis and systematic review. Heart Rhythm. 2018;15:1001–8.
Korutz AW, Obajuluwa A, Lester MS, McComb EN, Hijaz TA, Collins JD, et al. Pacemakers in MRI for the Neuroradiologist. Am J Neuroradiol. 2017;38:2222–30.
Mitka M. First MRI-safe pacemaker receives conditional approval from FDA. JAMA. 2011;305:985–6.
Celentano E, Caccavo V, Santamaria M, et al. Access to magnetic resonance imaging of patients with magnetic resonance-conditional pacemaker and implantable cardioverter-defibrillator systems: results from the really ProMRI study. EP Eur. 2018;20:1001–9.
Nazarian S, Roguin A, Zviman MM, Lardo AC, Dickfeld TL, Calkins H, et al. Clinical utility and safety of a protocol for noncardiac and cardiac magnetic resonance imaging of patients with permanent pacemakers and implantable-cardioverter defibrillators at 1.5 tesla. Circulation. 2006;114:1277–84.
•• Russo RJ, Costa HS, Silva PD, et al. Assessing the risks associated with mri in patients with a pacemaker or defibrillator. N Engl J Med. 2017;376:755–64 This is the first multicenter study that studies safety of MR unsafe CIEDs during MRI examinations. This is done while following the first MR examination protocol for patients with CIEDs.
Russo RJ. Determining the risks of clinically indicated nonthoracic magnetic resonance imaging at 1.5 T for patients with pacemakers and implantable cardioverter-defibrillators: rationale and design of the MagnaSafe registry. Am Heart J. 2013;165:266–72.
Horwood L, Attili A, Luba F, Ibrahim E-SH, Parmar H, Stojanovska J, Gadoth-Goodman S, Fette C, Oral H, Bogun F (2016) Magnetic resonance imaging in patients with cardiac implanted electronic devices: focus on contraindications to magnetic resonance imaging protocols. Europace euw122.
Shah AD, Patel AU, Knezevic A, et al. Clinical performance of magnetic resonance imaging conditional and nonconditional cardiac implantable electronic devices. Pacing Clin Electrophysiol. 2017;40:467–75.
•• Nazarian S, Hansford R, Rahsepar AA, Weltin V, McVeigh D, Gucuk Ipek E, et al. Safety of magnetic resonance imaging in patients with cardiac devices. N Engl J Med. 2017;377:2555–64 This is another major study that evaluated safety of MR unsafe CIEDs during MRI examinations.
Gopinathannair R, Mar PL, Gandhi G, Leiserowitz A, Tripuraneni A, Lakkireddy D, et al. Incidence and predictors of MRI scan utilization in MRI-conditional pacemaker recipients: a multicenter experience. Pacing Clin Electrophysiol. 2018;41:1519–25.
Verma A, Ha ACT, Dennie C, Essebag V, Exner DV, Khan N, et al. Canadian Heart Rhythm Society and Canadian Association of Radiologists consensus statement on magnetic resonance imaging with cardiac implantable electronic devices. Can Assoc Radiol J. 2014;65:290–300.
Lowe MD, Plummer CJ, Manisty CH, Linker NJ. Safe use of MRI in people with cardiac implantable electronic devices. Heart. 2015;101:1950–3.
Epstein Andrew E, DiMarco John P, et al. ACC/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities. Circulation. 2008;117:e350–408.
Sommer T, Naehle CP, Yang A, Zeijlemaker V, Hackenbroch M, Schmiedel A, et al. Strategy for safe performance of extrathoracic magnetic resonance imaging at 1.5 tesla in the presence of cardiac pacemakers in non–pacemaker-dependent patients: a prospective study with 115 examinations. Circulation. 2006;114:1285–92.
Katritsis DG, Hossein-Nia M, Anastasakis A, Poloniecki J, Holt DW, Camm AJ, et al. Myocardial injury induced by radiofrequency and low energy ablation: a quantitative study of CK isoforms, CK-MB, and troponin-T concentrations. Pacing Clin Electrophysiol. 1998;21:1410–6.
Higgins JV, Watson RE, Jaffe AS, Dalzell C, Acker N, Felmlee JP, et al. Cardiac troponin T in patients with cardiac implantable electronic devices undergoing magnetic resonance imaging. J Interv Card Electrophysiol Int J Arrhythm Pacing. 2016;45:91–7.
Mollerus M, Albin G, Lipinski M, Lucca J. Cardiac biomarkers in patients with permanent pacemakers and implantable cardioverter-defibrillators undergoing an MRI scan. Pacing Clin Electrophysiol. 2008;31:1241–5.
Padmanabhan D, Kella DK, Mehta R, Kapa S, Deshmukh A, Mulpuru S, et al. Safety of magnetic resonance imaging in patients with legacy pacemakers and defibrillators and abandoned leads. Heart Rhythm. 2018;15:228–33.
Martin ET, Coman JA, Shellock FG, Pulling CC, Fair R, Jenkins K. Magnetic resonance imaging and cardiac pacemaker safety at 1.5-tesla. JACC. 2004;43:1315–24.
Nitz WR, Brinker G, Diehl D, Frese G. Specific absorption rate as a poor indicator of magnetic resonance-related implant heating. Investig Radiol. 2005;40:773–6.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Jessica A. Martinez and Daniel B. Ennis declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Molecular Imaging.
Rights and permissions
About this article
Cite this article
Martinez, J.A., Ennis, D.B. MRI of Patients with Cardiac Implantable Electronic Devices. Curr Cardiovasc Imaging Rep 12, 27 (2019). https://doi.org/10.1007/s12410-019-9502-8
Published:
DOI: https://doi.org/10.1007/s12410-019-9502-8