Abstract
To investigate how soil fertilization/amendments alters arsenic speciation grain off-take in paddy rice, rice was grown to maturity in growth chambers fertilized with standard mineral fertilizer, wood ash (rich in silica), pig slurry (rich in organic matter), and non-amended control. The soil was sourced from a Chinese paddy field. The primary fertilized elements (nitrogen, phosphorus, potassium) were kept constant across treatments. Porewater chemistry and soil microbiology were monitored throughout the experiments. Total grain arsenic, sum of inorganic arsenic and dimethylarsinic acid (DMA), was significantly different between treatments (P = 0.024), with inorganic arsenic varying from 0.025 to 0.08 mg/kg and DMA from 0.08 to 0.16 mg/kg for control compared to fertilized, respectively. Fertilizer source made no difference to arsenic speciation concentration in grain. Porewater analysis found that as anaerobism set in, inorganic arsenic, phosphorus and manganese greatly lowered in concentration. Methylated arsenic species concentrations increased over time, concurrent with an increase in pH, decrease in Eh, and increase in total organic carbon and iron, with no strong treatment effects, except for pig slurry that enhanced pH and decreased Eh. Methanogenic archaea, sulfate-reducing bacteria and Acidobacteria increased with time and some Actinobacteria and Firmicutes increased due to slurry, but then decreased with time (P < 0.01). Methanogenic archaea and sulphate-reducing bacteria correlated positively with porewater DMA and negatively with porewater inorganic arsenic (P < 0.05). Genera within the Actinobacteria and Burkholderiaceae correlated negatively with DMA, while genera with iron-reducing capacity (Clostridiales) correlated positively with porewater inorganic arsenic and DMA (P < 0.05).
Similar content being viewed by others
Introduction
Compared to all other staple crops paddy rice is elevated in ~ tenfold in inorganic arsenic, the sum of arsenate and arsenite, which are both carcinogens (Carey et al. 2019). Rice grain elevation in inorganic arsenic is due to the cultivation of rice in anaerobic soils which leads to arsenic being mobilized through the conversion of relatively insoluble arsenate to more soluble arsenite (Afroz et al. 2019; Arao et al. 2009). Arsenate and arsenite are both efficiently assimilated by rice roots as they are phosphate and silicic acid analogues, respectively (Meharg and Zhao 2012). The biogeochemistry of arsenic in soils is complex as microbes may methylate arsenic species, though a series of redux ad methylation steps, conducted using arsenic reductase (arsC) and arsenic methyl transferase (arsM) proteins (Afroz et al. 2019; Li et al. 2014; Yang et al. 2018; Zhao et al 2013a,b), to give mono- through to tetra- ions. The di- form, dimethylarsinic acid (DMA), is found at high concentrations in grain. DMA is less efficiently assimilated by roots than arsenate or arsenite (Abedin et al. 2002), but is readily translocated within the plant to shoot (Raab et al. 2007).
Grain total arsenic, sum of inorganic arsenic and DMA, concentrations vary ~ 2-orders of magnitude globally (Carey et al. 2019). Soil arsenic concentrations are the key driver in arsenic assimilation into rice grain as these two parameters are well correlated (Adomako et al. 2008; Lu et al. 2009). Also, along with total arsenic, the relative contribution of inorganic arsenic and DMA vary greatly in soils, with DMA being very low at lower latitudes and elevated, sometimes more than 50% of sum of species, at higher latitudes (Carey et al. 2019; Dai et al. 2022). However, the partitioning between inorganic arsenic and DMA content of grain is less well understood. DMA is produced in soil through microflora and not in planta (Lomax et al. 2012). DMA is generated in soils through the plants life-cycle (Jia et al. 2015; Li et al. 2009; Liu et al. 2014; Ma et al. 2014; Norton et al. 2013; Xu et al. 2008). Thus assimilation into plant tissues must consider this. As well as temporal regulation of DMA accumulation in soils, arsenic cycling, in general, and DMA production in particular, can be highly dependent on other elements cycling, primarily fertilization with (organic) carbon (Ma et al. 2014; Norton et al. 2013), nitrogen (Ma et al. 2014) and silicon (Li et al. 2009; Liu et al. 2014). DMA can be produced aerobically and anaerobically, but in paddy soils it is anaerobic conditions that dominate DMA production (Arao et al. 2009; Jia et al. 2014, 2015; Li et al. 2009; Liu et al. 2014; Meharg and Zhao 2012; Norton et al. 2013; Wang et al. 2019; Xu et al. 2008; Zhao et al. 2013a). More reducing conditions, fuelled by organic matter as microbial growth substrate tends to enhance DMA production (Afroz et al. 2019; Norton et al. 2013).
Here, to understand more fully the dynamics of arsenic uptake into rice and translocation to grain, this study reports how soil porewater chemistry and soil microbiology evolves over the rice plants lifecycle under different organic and inorganic management regimes. Arsenic species and key elements are known to interact with arsenic biogeochemistry (carbon, manganese, iron, phosphorus), Eh and pH. So these parameters were made concurrently for an extracted porewater time-series, and the chemical elements in shoot and grain at harvest. Soil DNA was extracted at the same timepoints for monitoring of temporal fluctuations of the soil microbial diversity using 16S (bacteria, archaea) and ITS (fungi) amplicon sequencing. Results were interpreted within the context of sustainable production of rice with low arsenic for consumers.
Materials and Methods
Experimental Setup
Soil and rice seed (Yongyou12, a japonica-indica high yielding hybrid) were imported (under license from the Department for Environment and Rural Affairs, Northern Ireland) from the peri-urban catchment of Ningbo in S.E. China, (120°55″-122°16″E and 28°51″-30°33″N). Mixed household ash (from domestic hearth fuel) was obtained from Bangladesh (Mymensingh), and pig slurry from AFBI Hillsborough, Northern Ireland. At the start of the experiment the nutrient content of soil, ash and slurry were characterised (Table S1) for calculation of application rates. Total arsenic concentration in the soil was 4.66 ± 0.15 mg/kg, for pig slurry this figure was 0.36 ± 0.03 mg/kg, and ash < 3 mg/kg.
Rice seeds were germinated in the dark on wet tissue lined Petri dishes at 30˚C in an environmental growth cabinet (Fitotron, UK). After 2 weeks of germination seeds were transferred into a seed bed of soil filled modular plugs, measuring 3.7 × 3.7 × 4.5 cm, with 24 cells seed tray. The soil was mixed with sufficient water to saturate, and then fertilized at a rate of 1.125 g urea, 1.125 g KCl per 1 kg of soil. Two seedlings were planted in each plug and then the tray was placed in growth cabinet under a 14 and 10 d and night cycle with a corresponding temperature of 28 ˚C day and 23 ˚C night. Seedlings were then transplanted into a polypropylene container measuring 21 × 15 × 10 cm (Lakeland, Amazon.co.uk) which contained 1.2 kg of soil. The soils received 5 fertilizer treatments with 4 replicates per treatment. All treatments, with the exception of the control, were fertilized at a field application rate of 178.25 kg N/ha, 20.95 kg P/ha and 134.60 kg K/ha, though these were either from mineral fertilizer, household ash or pig slurry, with the latter 2 amended with mineral fertilizer to make up the difference. In Bangladesh, farmers typically apply 1 t of ash per hectare (1000 kg per 1,000,000 kg soil) (Gupta et al. 2023) therefore we applied 1.2 g mixed household ash per 1.2 k g soil. The ash used provided 0.012 g P per g ash, 0.050 g K per g ash and 0.26 g Si per g ash. The half-slurry treatment was 49.7 ml of liquid slurry which resulted in 89 kg N/ha, 10.5 kg P/ha and 67.3 kg K/ha, ~ half that of the mineral fertilizer treatment.
Ash, half-slurry (half-slurry plus half mineral fertilizer) and full-slurry-treatments were added at the time of transplanting rice. The mineral fertilizer and half-slurry-treatments had split mineral additions at 15 d post transplanting and at heading stage. Urea, calcium di-hydrogen phosphate; and potassium chloride were used as the source of these nutrients. One healthy rice seedling was transplanted in each container and grown at 25/23 ˚C for 14/10 h day-night cycle. To collect soil porewater a 10 cm Rhizon sampler (Rhizosphere Research, Netherlands) was inserted at a 45° angle through the centre of the growth container. The plants were watered regularly to maintain a 2 cm head of water on top of the soil using by Mili-Q water system to the grain-fill stage. After that soil was allowed to dry out to until harvest.
Porewater samples were collected at day 7 (L2), 14 (L3), 28 (L4), 56 (L5), 84 (L6) after transplanting, during the heading stage (HS, from day 99), during grain-fill stage (GF, from day 107) and after soil drying when grain was ripe (DR, from day 145).
Immediately after the collection of the pore water pH and Eh were measured, and 5 ml pore water was acidified using 50 ul concentrated nitric acid which was then stored in fridge at − 80˚C for the analysis of arsenic species and elemental content. Immediately after the collection of the porewater from all time points, soil samples were also collected from four sides of the pot, 5 cm from the stem at 90° to each other, using a 2 cm diameter cork-borer sampling core, and these samples were then mixed and 0.5 g soil was preserved for moisture and chemical analysis, and ~ 2.0 g soil was placed in a 2 ml Eppendorf tube and preserved immediately under the liquid N2 container and then transferred to a − 80 ˚C freezer for subsequent DNA extraction. Remaining soil was returned to the pot.
During the grain-fill, at the 12th d post-anthesis for each plant, the flag leaf and the most basal leaf of the first panicle were sampled. During drying stage, just before harvest, leaf and grain were also sampled. Leaf samples were cleaned with deionized water, excess water was dried using a paper towel and then cut into small pieces with clean scissors.
Chemical Analysis
The methodologies reported here are briefly summarized as they are covered in detail elsewhere (Signes-Pastor et al. 2016; Savage et al. 2017, Carey et al. 2018). pH and Eh of porewaters were measured using a calibrated pH meter, pHenomenal PH 1000 H (VWR International, UK. For Total Organic Carbon (TOC) analysis, 5 ml per sample was transferred to weighed sterile 15 ml falcon tubes and 50 µl 69% Analar grade HNO3 (Merck, Sigma-Aldrich, USA) was added. Samples were then run on a LOTIX TOC combustion analyser (USA).
To determine arsenic speciation of porewaters, 0.5 ml of pore water sample was diluted tenfold by adding 4.5 ml of 1% HNO3, made from 69% HNO3. Then, to 700 µl of the diluted porewater 7 µl of Prolabo Analar Normapur hydrogen peroxide, 30% (Prolabo, UK), was pipetted and mixed thoroughly. Samples were then run with standards prepared from a 100 µg/L DMA stock solution. A Thermo Dionex IC5000 Ion Chromatograph system, fitted with a Dionex IonPac AS7 RFIC analytical column (2 × 250 mm) and a Dionex AG7 guard column, was used to separate arsenic species. The mobile phase A contained 20 mM ammonium carbonate in deionised water and the mobile phase B contained 200 mM ammonium carbonate in deionised water were used. The flow rate for the run was 0.3 ml/min using the following gradient programme: 100% mobile phase A when time = 0 min, followed by a linear change to 100% mobile phase B when time = 10 min and finally followed by a linear change to 100% mobile phase A when time = 10.5 min, followed by 2 min equilibration, total analysis time 12.5 min. The ICP-MS (Thermo Scientific iCap Q) operating conditions were: Forward RF power- 1550 W; Nebuliser gas flow- ~ 1L/min, Nebuliser sample flow rate- ~ 0.35 ml/min. Helium was used as a collision gas at a flow rate of 4.5 ml/min., and the sole element measured was arsenic at a mass of 75.
Total elemental analysis of rice porewater was conducted on the same sample preparation used for arsenic speciation, but with 5 µl of 1000 µg/L rhodium internal standard (Fluka Analytical, Sigma-Aldrich, USA) added. ICP-MS was calibrated with ‘Multi-Element 2’ (SPEX CLMS-2 Multi-Element Solution 2, matrix: 5% HNO3) and ‘Multi-Element 4’ (SPEX CLMS-4 Multi-Element Solution 4, matrix: water/Tr-HF) standards which was connected to the auto-sampler.
All rice leaf, grain, soil and slurry were freeze-dried for 24 h using a Christ Alpha 1-4LD Plus freeze dryer, and grain manually dehusked. Dehusked grain and shoot were powdered using a Retsch PM100 Ball Mill (Retsch, Germany) fitted with zirconium dioxide vessel and grinding balls. Three replicates of 100 mg certified reference material (CRM), rice flour NIST SRM 1568b rice flour, soil CRM- NCS ZC73007 (CNAC, China) and mixed Polish Herb-INCT-MPH-2 (INCT, Poland) were used for the total elemental analysis, was run with each batch, as appropriate. To digest the plant material 10 ml of 1% Arsitar HNO3 was added to ~ 100 mg of powdered tissue, accurately weighed and microwave digested using a CEM Mars 6 1800W (USA). The programme used gradually heated the samples through a 3-stage process over a period of 35 min to 95 °C, and then sat at 95 °C for 30 min. After microwaving the digestate was centrifuged at 3500 rpm for 15 min, and then diluted to 10 ml with 1% HNO3. Arsenic speciation was then conducted on the diluted digestate using the same procedures as for porewaters. For total elemental analysis the same procedures were followed for digestion except that 2 ml of 69% Aristar HNO3 was added in each to digestion tubes, and then left to sit overnight. Then 2 ml of 30% Analar Normapur hydrogen peroxide (Prolabo, UK) was added to each tube and tubes were left open for 15 min before microwave digestion. On cooling, to each sample 30 µl of rhodium internal standard (Fluka Analytical, Sigma-Aldrich, USA) was added to each sample. Each tube was then made up to a final volume equivalent to 30 ml with deionised water. Total elemental analysis was then conducted by ICP-MS as per porewaters.
CRM recoveries for reported analytical determinants are given in Table S2. All reported analytes had a recovery between 71 and 101%. The chemical data were tested for normality and if not normally distributed the data transformed into ranked data or a non-parametric test applied. This was followed by Principal Component Analysis (PCA), General linear modelling (GLM), linear regression, correlation analysis and generation of plots (Minitab 19, GraphPad Prism 8.0).
Molecular Analysis
DNA was extracted from 0.5 g of soil collected from each pot at day 14, 56, GF and DR using the Powerlyser Power soil DNA extraction kit (Qiagen, UK) following the manufacturer’s protocol. Bead beating was performed with a Precellys Dual (Bertin technologies, France) at 3 × 6000 rpm for 40 s. In between cycles, the tubes were kept on ice for 1 min. DNA quality was assessed on an agarose gel and with NanoDrop 8000 spectrophotometer (Thermo Fisher Scientific, UK). Following this, 300 bp paired end amplicon sequencing was performed on the Illumina Miseq, using the standard Illumina 2-step protocol (Caporaso et al. 2011) for generation of amplicon libraries, with target specific primers for 16S (515F/806R, Caporaso et al. 2011) and fungal ITS (ITS1F/ITS2R, Mello et al. 2011; White et al. 1990). The raw fastq files generated for 16S and ITS amplicons from each DNA sample were submitted to the European short read archive (ENA) and can be accessed under accession number PRJEB45605 (16S time-course data) and PRJEB45610 (ITS time-course data).
Bioinformatics and Statistical Analysis
The amplicon data were processed with Qiime2 (Bolyen et al. 2019), using dada2 (Callahan et al. 2016) for generation of the amplicon sequence variant table (ASV table). The 16S sequences were annotated against the silva (version 138 SSU) (Quast et al. 2012) and the ITS sequences against the unite (version8, 04/02/2020) database (Nilsson et al. 2019) using the blast consensus option. Qiime2 generated count table and taxonomy files were exported and used for downstream analysis and plotting in R v4.1.1 with R packages phyloseq v1.36.0 (McMurdie and Holmes 2013), DESeq2 v1.32.0 (Love et al. 2014), pheatmap v1.0.12 (Kolde 2019) and ggplot2 v3.3.6 (Wickham 2016). Phylum and genus level relative abundance (RA) plots of the 16S and ITS amplicon data were generated with phyloseq and ggplot2, depicting RA of annotated ASVs with median RA > 1% and the rest summed up under others. DESeq2 analysis was employed at genus level for generation of a normalised count table and identification of significant time and/or treatment effects using the LRT test (Time + Treatment + Time*Treatment) and WALD test (3 pairwise comparisons for time: L3 versus L5, GF, DR; 4 pairwise comparisons for treatment: C versus A, FS, HS, MF). Following this, genus level results were compiled, genus level ASVs with overall basemean < 10 removed, and a heatmap generated (mean centred normalised counts) with R package pheatmap, for genus level ASVs with adjusted P value < 0.01 (LRT test), that were also annotated at genus level.
For integration of chemical porewater and soil microbial data, Spearman correlation analysis with R library Hmisc, corrplot using R function rcorr and corrplot was employed (Wei and Simko 2021). Spearman correlation analysis was performed between the normalised count of all genus level ASVs with basemean > 10 and the chemical data. The Spearman correlation R and P values generated were integrated into the overall results table. Genus level ASVs with Spearman correlation P value < 0.001 for inorganic arsenic, DMA, MMA or TMAO were identified and correlation plots generated for the subset of those that were also annotated at genus level.
Results
Chemistry
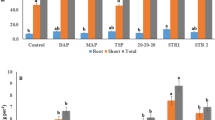
At harvest, no significant treatment effect (P > 0.05) was identified for rice biomass with respect to shoot or grain weight (Fig. 1). Per pot, root biomass dry weight means by treatment varied between 13 and 15 g, straw between 37 and 52 g, and husked grain between 8 and 13 g.
From grain GF and SD sampling points, grain inorganic arsenic varied from 0.025 to 0.08 mg/kg (Fig. 1), grain DMA from 0.08 to 0.16 mg/kg, shoot inorganic arsenic was around 2.5 mg/kg and shoot DMA around 0.05 mg/kg. Multivariate, inorganic arsenic and DMA, GLM analysis found that grain inorganic arsenic differed significantly between treatments (P = 0.024). This significant difference was due to the fact that all fertilized treatments had greater inorganic arsenic than the control, ~ 2–3 times greater. Grain inorganic arsenic was greater at SD as compared to GF, but this was not significantly so, and there was also no significant interaction, both P > 0.05. Grain DMA was significantly greater (P < 0.0001) than inorganic arsenic, by ~ threefold across all treatments. No treatment DMA terms were significant for grain. For shoot (Fig. 1), no terms in for the multivariate GLM were significant (P > 0.05) except between inorganic arsenic and DMA concentration (P < 0.0001).
Porewater pH, Eh, TOC, inorganic arsenic, MMA, iron, phosphorus and manganese showed a highly significant response (P < 0.001) to time, while DMA (P = 0.004) and TMAO (P = 0.001) were also significant for this term (Fig. 2). For all reported analytes, neither treatment or treatment*time was significant in the GLM, with the exception of inorganic arsenic where there was a significant treatment effect (P = 0.026). The porewater inorganic arsenic concentrations for the control treatment were generally lower, and less variable, than for all other treatments (Fig. 2). At only 2 same points, 7 and 28 d, was any other treatment lower in porewater inorganic arsenic than the control, and on both these dates it was the mineral fertilizer treatment, and porewater concentrations were only slightly lower here than for the control treatment. Over time, porewater Eh, inorganic arsenic, P and Manganese decreased, while the pH increased. For inorganic arsenic there was a sharp decrease at the final time point for all treatments, relating to when the soils were dry at harvest, though Eh had not responded to the onset of more aerobic conditions. TOC, iron, MMA, DMA, and to a lesser extent TMAO, concentrations started out relatively low and then rose between 40 and 80 d, staying elevated until the onset of drying at the final time point. TOC and iron exhibited the most dramatic changes, rising 3–fourfold from the initial measurement to their maximum concentration. This change in TOC, iron and methylated arsenic species occurred when Eh changed from positive to negative, showing the onset of more reducing conditions accompanied by alkalinisation of porewater. Hence porewater TOC and iron may be of potential significance to methylation of As, and accumulation of MMA into rice shoot and DMA into rice grain. Furthermore, with respect to treatment, for Eh and pH, the strongest differences resulted from the full-slurry addition, followed by the half-slurry, resulting in more negative Eh and higher pH, with these differences becoming more pronounced as the experiment proceeded. Full-slurry followed by half-slurry also resulted in higher levels of inorganic arsenic, in particular at the start of the experiment, where slurry-treatments showed ~ double the inorganic arsenic in pore waters compared to other treatments.
Chemistry of porewaters over the entire plant growth cycle. The treatement abbreviations are as those for Fig. 1
The complexity of element interactions/correlations are shown in a progression of Spearman’s Rank correlations at each time of porewater sampling times is shown in Fig. 3. This time-series progression shows generically that the elements are arsenic species are positively correlated with each other, with these correlations starting to degrade a bit by GF, and breaking down further at SD. With respect to pH and Eh, there is an interesting inversion of relationships as time progresses. At day 7 pH is positively correlated with all arsenic species and elements, but degrades to neutral by day 28 and then shifts to negative correlations by day 56 and stays this way until anaerobism is enhanced at soil drying. Eh shows the opposite progression, as would be expected as Eh and pH are highly negatively correlated (Fig. 3). Overall, i.e., integrating all timepoint, significant correlations (P < 0.05) are show in Figure S1 and Figure S2. The negative associations of methylated species concentrations with pH, and the positive with Eh and TOC are illustrated in Figure S1, while the positive correlations between all arsenic species are shown in Figure S2.
Microbial Communities
Relative Abundance
The most abundant phyla identified within each kingdom were Firmicutes, Proteobacteria, Actinobacteria (bacteria), Euryarchaeota (archaea), Ascomycetes (fungi) with bacteria showing the highest level of diversity (Fig. 4a and Fig. 4c). There were 18 bacterial genera level annotated ASVs identified at > 1% median relative abundance with Nocardioides (phylum Actinobacteria), Defluviicoccus (phylum Proteobacteria), Bacillus (phylum Firmicutes), Aquisphaera (phylum Planctomycetes), Clostridium sensu stricto 10 (phylum Firmicutes), Mycobacterium (phylum Actinobacteria) the top most abundant, while Methanobacterium and Methanosarcina were the most abundant archaeal genera (Fig. 4b, additional file 2). There were 7 fungal genera level annotated ASVs identified at > 1% median relative abundance (Fig. 4d). Most fungal sequences (80%) mapped to 3 genus level ASVs, but only 2 of these, Talaromyces (11%) and Morteriella (10%) were annotated at genus level (Fig. 4d). The most abundant genus level fungal ASV (59%) was an Ascomycete within the order Thelebolales that was not annotated at family or genus level and hence absent in Fig. 4d (additional file 2).
Relative abundance plots of the amplicon data. A: 16S phylum level, B: 16S genus level, C: ITS phylum level, D: ITS genus level. For genus level ASVs, shows only those that are also annotated at genus level. Label = median relative abundance of replicates (n = 4) for Treatment_Time. Treatment: C control, A ash, FS full-slurry, HS half-slurry; Time: L3 = day 14, L5 = day 56, GF grain-fill stage, DR drying stage
Time and/or Treatment Effect on Community Composition
There were 172 genus level 16S ASVs (80 of these annotated at genus level) and 9 genus level ITS ASVs (4 of these annotated at genus level) with identified significant changes in response to time, treatment and/or time*treatment (adjusted P value < 0.01 for LRT test), with very clear effects observed for bacteria and archaea, but not for fungal ASVs. Most of the significant genus level 16S ASVs (136) increased over time, while 21 decreased. A total of 23 genus level 16S ASVs (16 of these annotated at genus level) responded to treatment (adjusted P value < 0.01 for LRT test and relevant Wald tests), of these 14 also showed a significant time effect. The strongest treatment response was an increase of some bacterial genus level ASVs (15) in response to slurry treatment, with 8 of these also showing a significant decrease in abundance over time (additional file 2). There was an increase over time in genus level ASVs within the phylum Euryarchaeota (Methanobacterium, Methanocella, Rice cluster 1, Methanoregula, Methanospirillum, Methanosaeta), within the class Anaerolinea (Anaerolinea, Leptolina), within the phylum Acidobacteria (Koribacter, Solibacter, Palaudibaculum, Geothrix), and for sulfate-reducing bacteria within the phylum Proteobacteria (Syntrophobacter, Desulfovibrio, Desulfatiglans), while genus level ASVs within the phylum Actinobacteria (Mycobacterium, Acidothermus, Streptomyces) decreased (Fig. 5a). Furthermore, there was a significant increase in response to slurry treatment of genus level ASVs annotated as Sarcina, Alkaliphilus, Intestinibacter, Terrisporobacter, Turicibacter (phylum Firmicutes) and Fermentimonas, Proteiniphillum (phylum Bacteroidetes), with these subsequently decreasing over time (Fig. 5a). However, no convincing time and/or treatment effect was observed for significant genus level annotated ITS ASVs (Fig. 5b). For full detail on the 16S and ITS genus level abundance data and DESeq2 result see additional file 2.
Heatmap of genus level 16S and ITS ASVs that show significant time, treatment or time*treatment effect (LRT test, adjusted P value < 0.01) and are annotated at genus level. Label = Time_Treatment_Replicate. Time: L3 = day 14, L5 = day 56, GF = grain-fill stage, DR drying stage; Treatment: C control, A ash, FS full-slurry, HS half-slurry; Replicate 1,2,3,4
Spearman Correlation of Microbial ASVs with Arsenic Species in Porewater
Highly significant Spearman correlation (P < 0.001) with porewater arsenic species (iAs, DMA, MMA or TMAO) was identified for 240 of the 16S genus level ASVs, 125 of these annotated at genus level (Fig. 6). Methanogenic archaea (Methanobacterium, Methanocella, Methanospirillum, Methanosarcina, Rice cluster 1) as well as sulfate-reducing bacteria within the phylum Proteobacteria (Syntrophobacter, Desulfovibrio, Desulfatiglans) were significantly (Spearman P < 0.05) positively correlated with MMA and pH (in some instances also DMA). Most of these were also positively correlated with TOC and pH and negatively correlated with Eh and iAs (Fig. 6) as well as showing a significant increase in abundance over time (LRT test and WALD tests for time effect, adjusted P value < 0.01) (Fig. 5a). The methanogenic archaea Methanomassiliicoccus showed significant (Spearman P < 0.05) correlation with MMA (Fig. 6), but did not significantly increase with time. Firmicutes within the order Clostridiales with iron and/or sulphate-reducing capacities (i.e., Clostridium sensu stricto 3, Clostridium sensu stricto 8, Clostridium sensu stricto 10, Clostridium sensu stricto 13, Desulfitobacterium, Desulfosporosinus, Desulfotomaculum, Oxobacter, Anaerovorax, Gracilibacter, Lutispora) were strongly positively correlated with inorganic arsenic, DMA, MMA, phosphorus and manganese and showed no significant correlation with pH or Eh (Fig. 6) and did not change abundance in response to time and/or treatment (LRT test, adjusted P value > 0.05, additional file 2). With respect to the genera within the phylum Firmicutes (Sarcina, Alcaliphilus, Intestinibacter, Romboutsia, Sporacetigenium, Turicibacter, Terrisporobacter), which significantly increased in response to slurry treatment (Fig. 5a, LRT test and WALD tests for slurry effect, adjusted P value < 0.01), most showed no strong significant correlation with porewater arsenic species and are therefore absent in Fig. 6. Romboutsia showed weak positive correlation with MMA and inorganic arsenic (P < 0.05). Sporacetigenium showed weak positive correlation with MMA, (P < 0.05), and strong positive correlation DMA (P < 0.001). In contrast to this, 16 genus level annotated ASVs within the phylum Actinobacteria (Jatrophihabitans, Geodermatophilus, Augustibacter, Cellulomonas, Oryzihumus, Phycicoccus, Terrabacter, Pseudarthrobacter, Actinoplanes, Marmoricola, Nocardioides, Aestuariimicrobium, Microlunatus, Actinomycetospora, Pseudonocardia, Actinomadura) and 6 genus level annotated ASVs within the family Burkholderiaceae (Burkholderia, Herminiimonas, Massilia, Noviherbaspirillum, Ramlibacter, Variovorax) showed significant negative correlation with DMA and MMA (Spearman P < 0.05), but no significant correlation (Spearman P > 0.05) with Eh or pH, (Fig. 6). With the exception of Pseudonorcadia, Actinomadura, none of these changed significantly in abundance in response to time or treatment (LRT test, adjusted P value > 0.01) and are hence absent in Fig. 5a. None of the genera level fungal ASVs showed strong correlations with porewater arsenic species and are hence absent in Fig. 6. For full detail on the 16S and ITS genus level Spearman’s rank correlation results see additional file 2.
Spearman correlation plot between porewater chemical (key drivers in arsenic biogeochemistry) and microbial genus level amplicon data. Shows results for genus level annotated ASVs that are strongly correlated (Spearman P value < 0.001) with iAs, MMA, DMA or TMAO. A shows all correlations; B shows only significant correlations (Spearman P value < 0.05, which equates approx. to a Spearman correlation Rvalue of either > 0.24 or < − 0.24). Colour scale ranges from pink (Spearman correlation Rvalue = 1) to black (Spearman correlation Rvalue = − 1), with white indicating no correlation (Spearman correlation R value = 0)
Discussion
Porewater arsenic chemistry was highly dynamic with time. Inorganic arsenic decreased in porewaters sharply at onset of flooding, and then still further, after a period of plateauing, on soil drying. This pattern is similar to the steep decline in manganese, and very similar to that of phosphorus. Manganese is known to form oxides in the rice rhizosphere, due to radial oxygen loss, leading to immobilization of arsenate and phosphate (Chen et al 2006; Liu et al. 2005). DMA initially deceased to day 40, increased and peaked at day 120, before failing on soil drying, while MMA simply increased to day 120, and decreased on soil drying. TMAO showed the same general pattern as DMA, but are lower concentrations. The increase to day 120 in methylated species very much follows the TOC and iron pattern, and indeed TOC and iron in porewaters correlate well with methylated arsenic species. There were large treatment driven shifts in Eh and pH, particularly when comparing full-slurry treatment to mineral fertilizer. Eh settled at − 20 and − 40 mV, pH at 7.5 and 7 for full-slurry and mineral fertilizer, respectively. Redox also steadily drops to day 120, and this it appears that methylation is related to TOC driven negative redox change, as generally observed in the literature (Aaro et al. 2009; Jia et al. 2014; Zhao et al. 2013a, b). With increased duration of flooding, the Eh decreased while at the same time the pH increased. This trend was most pronounced in the full-slurry treatment. As to be expected, this was accompanied by a significant change in the microbial community characterised by an increase in methanogenic archaea and sulphate-reducing bacteria (anaerobes) and decrease in some Actinobacteria (aerobes) over time, which can be attributed to the competitive advantage of anaerobes at the increasingly lower Eh.
Soil microbes responded mainly to slurry treatment, which resulted in a significant increase in genus level ASVs within the phylum Firmicutes, most of these decreasing over time. As the microbiota of pig slurry has been reported to contain 75% Firmicutes and 14% Bacteroidetes (Kumar et al. 2020), it seems likely that these were introduced or significantly stimulated by the slurry treatment, but subsequently unable to persist over time due to competition with the in situ soil flora. No effect of treatment was observed for archaeal or fungal genus level ASVs.
The biogeochemical processes that lead to arsenic mobilization in soils are dynamic and highly complex and are thought to involve microbial processes such as methylation/demethylation (Afroz et al. 2019), organic matter driven reduction in redox (Ma et al. 2014; Norton et al. 2013; Williams et al. 2011), nitrate utilization as a terminal electron acceptor (Ma et al. 2014), sulphate reduction (Jia et al. 2015) and reduction/oxidation of insoluble iron and manganese (Dai et al. 2022; Liu et al. 2005; Yang et al. 2018). Further to that, ionic methylated species (MMA, DMA, TMAO, tetramethylarsonium) can be lost from the system through both biovolatilization (after reduction) and demethylation (Chen et al. 2019). In the current study Spearman correlation analysis implies that arsenic methylation may be driven by methanogens within the phylum Euryarchaeota and bacteria with sulphate-reducing capacity (positive correlation mostly with MMA and for some organisms also with DMA). Previous studies support a role of sulphate-reducing bacteria in arsenic methylation (Chen et al. 2019; Wang et al. 2019, 2020). However, while Chen et al. (2019) initially investigated a role of sulphate-reducing bacteria and methanogenic archaea in arsenic methylation, results from their study suggest a role in methylation only for sulphate-reducing bacteria and in de-methylation of DMA to MMA for methanogenic archaea. As in the present study most of the sulphate-reducing bacteria and methanogenic archaea showed stronger correlation with MMA than with DMA, a direct role of both sulphate-reducing bacteria and methanogenic archaea in generation of MMA via methylation is the logical conclusion, although demethylation from DMA to MMA could also explain this result. The current study furthermore implies that bacteria with iron-reducing capacity within the phylum Firmicutes (positive correlation with inorganic arsenic, DMA and MMA) are important for arsenic dynamics in rice paddies, which is in agreement with previous studies, which identified a link between iron-reducing bacteria such as Clostridium sensu strictu (Phylum Firmicutes) and increased mobilisation of arsenic from iron plaque and concurrent increase in methylated arsenic species (Dai et al 2020, Yang et al. 2018, Wang et al. 2019). It is proposed that genera within the phylum Actinobacteria and within the family Burkholderiaceae (negative correlation with DMA and MMA) demethylate or decrease methylation of arsenic. There are few studies that link demethylation of arsenic to specific groups of microbes, and only methanogenic archaea have so far been implicated (Chen et al. 2019). Actinobacteria have been shown to produce arsenic binding siderophores (Retamal-Morales et al. 2018), and Burkholderiaceae have been linked to arsenic oxidation and immobilisation in shallow water (Sonthiphand et al. 2021) and these processes could indirectly facilitate the decrease in methylated arsenic species. However, a direct role of these organisms in demethylation of DMA and MMA should not be ruled out. Finally, while soil fungi with arsenic bioaccumulation and plant growth promotion capacity have been proposed for use in agriculture (Singh et al. 2015; Segura et al. 2018), in the current study rice paddy fungi were found to be dominated by a few genera, most within the phylum Ascomycetes, that were unresponsive to treatment or time and were not associated with arsenic species in porewater. However, while these results allow formation of interesting hypothesis with respect to the role of different groups of paddy soil microbes, as these conclusions are purely based on correlation analysis, follow-on studies should conducted to further explore these results. The most likely scenario is that microbial response to soil factors (TOC, iron, manganese, phosphorus) is interacting with arsenic’s biogeochemical cycle, where microbial processes drive inorganic arsenic porewater mobilization and transformation in porewater, which subsequently affect grain inorganic arsenic concentration.
In the experiments reported here DMA dominate grain speciation (3:1), but not shoot (1:10). This is to be expected as previous experiments have shown that DMA is efficiently translocated to filling rice grain (Carey et al. 2011). It is interesting that DMA was insensitive to fertilizer treatment, yet it is often linked to soil organic matter additions (Afroz et al. 2019; Norton et al. 2013; Zhao et al. 2013a), suggesting that redox could be the driver of methylation per se, as this was relatively consistent between treatments and progressed with time through the experiment. However, as slurry application here increased the abundance of some Firmicutes and as many Firmicutes with iron-reducing capacity (while not significantly increased in response to slurry) were positively correlated with porewater inorganic arsenic, DMA and MMA, and a small number of Firmicutes that were significantly increased in response to slurry were positively correlated with DMA, MMA and/or inorganic arsenic, this also shows a potential route for slurry application to increase inorganic arsenic, DMA and MMA via increase of arsenic methylating microbes as observed in response to manure (Afroz et al. 2019).
The reported study also highlights the potential importance of manganese in soil arsenic processes. Manganese shows the most dramatic and sustained reduction in porewater over time, in contrast to iron and TOC, although phosphate also follows, but less dramatically comparted to manganese. The manganese concentration in porewaters also tracks that of inorganic arsenic. Iron plaque has been extensively studied with respect to arsenic dynamics in the rhizosphere where they are formed due to radial oxygen release from roots converting soluble iron(II) of reduced bulk soil to insoluble iron (III) (Chen et al. 2006; Liu et al. 2005). While it is noted that manganese oxide plaques could be just as important for immobilizing inorganic arsenic, yet they are not well understood.
There was little contrast between in grain arsenic speciation between GF and SD, as the soil switches from anaerobic to aerobic conditions when irrigation ceases to prepare the ground and crop for harvesting (Arao et al. 2009). While there is a strong contrast between upland (more aerobic soils) and lowland (more anaerobic soils) in arsenic concentrations in the grain, with lowland being lower (Meharg and Zhao 2012), from the experiments presented here the switch to drying out soils did little, suggesting that the bulk of the arsenic that is to be transferred to the leaf is already in root or shoot tissue (Carey et al. 2011; Raab et al. 2007). This rules out soil drying at harvesting as a potential management technique to lower grain arsenic (Ma et al. 2014; Xu et al. 2008).
At harvest, addition of fertilizer, both mineral (NPK and ash) and organic (pig slurry), did not cause enhancement of plant biomass parameters. The lack of biomass enhancement may be an artefact from the fact that the plants were grown in relatively small volumes of soil in enclosed containers that did not have leaching. Also, although soil was from an actively managed paddy, it may have already been at sufficient nutrient supply for the plant biomass produced. This non-response to fertilizer amendment must be born in mind in interpreting the experiment. However, grain arsenic speciation did change, with an increase in grain arsenic compared to control (non-fertilized) treatment, although there was no effect of any treatment on rice biomass, shoot inorganic arsenic or DMA, or grain DMA. Thus the experiments reported here give an insight on how different fertilizer amendments alter arsenic speciation dynamics in the soil–plant system. The lower grain inorganic arsenic in the most infertile soil may explain a global trend that inorganic arsenic and total grain arsenic is lower in rice cultivated in the Southern Hemisphere, particularly for Africa and Asia (Carey et al. 2019; Dai et al. 2022). These soils tend to be more weathered and nutrient deficient, but also have lower fertilizer inputs, leading to lower yields that the global norm for rice. It appears that such low input systems may have the benefit for producing low inorganic arsenic rice grain, although total arsenic in the soil and its bioavailability also need to be considered.
Data Availability
Data generated or analysed during this study are included in this article and its supplementary information files. The raw fastq files generated for 16S and ITS amplicons from each DNA sample were submitted to the European short read archive (ENA) and can be accessed under accession number PRJEB45605 (16S time-course data) and PRJEB45610 (ITS time-course data).
References
Abedin MJ, Feldmann J, Meharg AA (2002) Uptake kinetics of arsenic species in rice (Oryza sativa L.) plants. Plant Physiol 128:1120–1128
Adomako EE, Soliaman ARM, Williams PN, Deacon C, Rahman GKMM, Meharg AA (2008) Enhanced transfer of arsenic to grain for Bangladesh grown rice compared to US and EU. Environ Int 35:476–479
Afroz H, Su SM, Carey M, Meharg AA, Meharg C (2019) Inhibition of microbial methylation via arsM in the rhizosphere: arsenic speciation in the soil to plant continuum. Environ Sci Technol 53:3451–3463
Arao T, Kawasaki A, Baba K, Mori S, Matsumoto S (2009) Effects of water management on cadmium and arsenic accumulation and dimethylarsinic acid concentrations in Japanese rice. Environ Sci Technol 43:9361–9367
Bolyen E, Rideout JR, Dillon MR, Bokulich NA, Abnet CC, Al-Ghalith GA et al (2019) Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat Biotechnol 37:852–857
Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJA, Holmes SP (2016) DADA2: high-resolution sample inference from Illumina amplicon data. Nat Methods 13:581–583
Caporaso JG, Lauber CL, Walters WA, Berg-Lyons D, Lozupone CA, Turnbaugh PJ et al (2011) Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc Natl Acad Sci 108:4516–4522
Carey AM, Norton GJ, Deacon C, Scheckel KG, Lombi E, Punshon T, Guerinot ML, Lanzirotti A, Newville M, Choi Y, Price AH, Meharg AA (2011) Phloem transport of arsenic species from flag leaf to grain during grain filling. New Phytol 192:87–98
Carey M, Donaldson E, Signes-Pastor AJ, Meharg AA (2018) Dilution of rice with other gluten free grains to lower inorganic arsenic in foods for young children in response to European Union regulations provides impetus to setting stricter standards. PLoS One 13(3):e0194700
Carey M, Meharg C, Williams P, Marwa E, Jiujin X, Gomes Farias J, De Silva PMCS, Signes-Pastor A, Lu Y, Nicoloso FT, Savage L, Campbell K, Elliott C, Adomako E, Green AJ, Moreno-Jimenez E, Carbonell-Barrachina ÁA, Triwardhani EA, Pandianga FI, Haris PI, Lawgali YF, Sommella A, Pigna M, Brabet C, Montet D, Njira K, Watts MJ, Meharg AA (2019) Global sourcing of low-inorganic arsenic rice grain. Exposure Health 12:711–719
Chen Z, Kim KW, Zhu YG, McLaren R, Liu F, He JZ (2006) Adsorption (AsIII, V) and oxidation (AsIII) of arsenic by pedogenic Fe-Mn nodules. Geoderma 136:566–572
Chen C, Li L, Huang K, Zhang J, Xie WY, Lu Y, Dong X, Zhao FJ (2019) Sulfate-reducing bacteria and methanogens are involved in arsenic methylation and demethylation in paddy soils. ISME J 13:2523–2535
Dai J, Jiang N, Kopittke PM, Zhao FJ, Wang P (2020) Increased arsenic mobilization in the rice rhizosphere is mediated by iron-reducing bacteria. Environ Pollut 263:114561
Dai J, Tang Z, Gao AX, Planer-Friedrich B, Kopittke PM, Zhao FJ, Wang P (2022) Widespread occurrence of the highly toxic demethylated monothioarsenate (DMMTA) in rice globally. Environ Sci Technol 56:3575–3586
Gupta Y, Hossain M, Islam MR, Talukder MMH, Khokon MAR, Uddun MM, Kabir H, Carey M, Raphs K, de Moraes NV, Meharg AA, Meharg C (2023) Recycled household ash in rice paddies of Bangladesh for sustainable production of rice without altering grain arsenic and cadmium. Expos Health. https://doi.org/10.1007/s12403-023-00539-y
Huang L, Wang X, Chi Y, Huang L, Li WC, Ye Z (2021) Rhizosphere bacterial community composition affects cadmium and arsenic accumulation in rice (Oryza sativa L). Ecotox Environ Safety. 222:112474
Jia Y, Huang H, Chen Z, Zhu YG (2014) Arsenic uptake in rice is influenced by microbe-mediated arsenic redox changes in the rhizosphere. Environ Sci Technol 48:1001–1007
Jia Y, Bao P, Zhu YG (2015) Arsenic bioavailability to rice plant in paddy soil: influence of microbial sulphate reduction. J Soils Sed 15:1960–1967
Kolde R (2019) R package pheatmap, pretty heatmaps (https://CRAN.R-project.org/package=pheatmap)
Kumar H, Jang YN, Kim K, Park J, Jung MW, Park JE (2020) Compositional and functional characteristics of swine slurry microbes through 16S rRNA metagenomic sequencing approach. Animals (basel) 10:1372
Li RY, Stroud JL, Ma JF, McGrath SP, Zhao FJ (2009) Mitigation of arsenic accumulation in rice with water management and silicon fertilization. Environ Sci Technol 43:3778–3783
Liu WJ, Zhu YG, Smith FA (2005) Effects of iron and manganese plaques on arsenic uptake by rice seedlings (Oryza sativa L.) grown in solution culture supplied with arsenate and arsenite. Plant Soil 277:127–138
Liu WJ, McGrath SP, Zhao FJ (2014) Silicon has the opposite effects on the accumulation of inorganic arsenic and methylated arsenic species in rice. Plant Soil 376:423–431
Lomax C, Liu WJ, Wu L, Xue K, XiongJ ZJ, McGrath SP, Meharg AA, Miller AJ, Zhao FJ (2012) Methylated arsenic species in plants originate from soil microorganisms. New Phytol 193:665–672
Love MI, Huber W, Anders S (2014) Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15:550
Lu Y, Adomako EE, Solaiman ARM, Islam RM, Deacon C, Williams PN, Rahman GKMM, Meharg AA (2009) Baseline soil variation is a major factor in arsenic accumulation in Bengal Delta paddy rice. Environ Sci Technol 43:1724–1729
Ma R, Shen J, Wu J, Tang Z, Shen Q, Zhao FJ (2014) Impact of agronomic practices on arsenic accumulation and speciation in rice grain parts. Environ Pollut 194:217–223
McMurdie PJ, Holmes S (2013) Phyloseq: An R package for reproducible interactive analysis and graphics of microbiome census data Watson M, editor. PLoS One. 8:e61217
Meharg AA, Zhao FJ (2012) Arsenic and rice. Springer, Amsterdam
Mello A, Napoli C, Murat C, Morin E, Marceddu G, Bonfante P (2011) ITS-1 versus ITS-2 pyrosequencing: a comparison of fungal populations in truffle grounds. Mycologia 103:1184–1193
Nilsson RH, Larsson KH, Taylor AFS, Bengtsson-Palme J, Jeppesen TS, Schigel D, Kennedy P, Picard K, Glöckner FO, Tedersoo L, Saar I, Kõljalg U, Abarenkov K (2019) The UNITE database for molecular identification of fungi: handling dark taxa and parallel taxonomic classifications. Nucleic Acids Res 47:D259–D264
Norton GJ, Adomako EA, Deacon CM, Carey AM, Price AH, Meharg AA (2013) Effect of organic matter amendment, arsenic amendment and water management on grain arsenic species. Environ Pollut 177:38–47
Quast C, Pruesse E, Yilmaz P, Gerken J, Schweer T, Yarza P et al (2012) The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res 41:D590–D596
Raab A, Meharg AA, Feldmann J (2007) Uptake and translocation of inorganic and methylated arsenic species by plants. Environ Chem 4:197–203
Retamal-Morales G, Mehnert M, Schwabe R, ischler D, Zapata C, Chávez R, Schlömann M, Levicán G (2018) Detection of arsenicbinding siderophores in arsenic-tolerating Actinobacteria by a modified CAS assay. Ecotoxicol Enviro Saf 157:176–181
Savage L, Carey M, Summon M, Islam R, de Silva M, Williams PN, Meharg AA (2017) Elevated trimethylarsine oxide and inorganic arsenic in northern hemisphere summer monsoonal wet deposition. Environ Sci Technol 51:12210–12218
Segura FR, Paulelli ACC, Braga GUL, Filho WRD, Silva FF, Batista BL (2018) Promising filamentous native fungi isolated from paddy soils for arsenic mitigation in rice grains cultivated under flooded conditions. J Environ Chem Engin 6:3926–3932
Signes-Pastor AJ, Carey M, Meharg AA (2016) Inorganic arsenic in rice-based products for infants and young children. Food Chem 191:128–134
Singh M, Srivastava P, Verma P, Kharwar R, Singh N, Tripathi R (2015) Soil fungi for mycoremediation of arsenic pollution in agriculture soils. J Appl Microbiol 119:1278–1290
Sonthiphand P, Rattanaroongrot P, Mek-yong K, Kusonmano K, Rangsiwutisak C, Uthaipaisanwong P, Chotpantarat S, Termsaithong T (2021) Microbial community structure in aquifers associated with arsenic: analysis of 16S rRNA and arsenite oxidase genes. PeerJ 9:e10653. https://doi.org/10.7717/peerj.10653
Wang J, Kerl CF, Hu P, Martin M, Mu T, Bruggenwirth L, Wu G, Said-Pullicino D, Romani M, Wu L, Planer-Friedrich B (2020) Thiolated arsenic species observed in rice paddy pore waters. Nat Geosci 13:282–287
Wang M, Tang Z, Chen XP, Wang X, Zhou WX, Tang Z, Zhang J, Zhao FJ (2019) Water management impacts the soil microbial communities and total arsenic and methylated arsenicals in rice grains. Environ Pollut 247:736–744
Wei T, Simko V (2021) R package “corrplot”: visualisation of a correlation matrix. https://github.com/taiyun/corrplot
White TJ, Bruns T, Lee S, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR Protocol 18:315–322
Wickham H (2016) ggplot2: Elegant Graphics for Data Analysis. Springer-Verlag, New York
Williams PN, Islam S, Islam R, Jahiruddin M, Adomako E, Soliaman ARM, Rahman GKMM, Lu Y, Deacon C, Zhu YG, Meharg AA (2009) Arsenic limits trace mineral nutrition (selenium, zinc and nickel) in Bangladesh rice grain. Environ Sci Technol 43:8430–8436
Williams PN, Zhang H, Davison W, Meharg AA, Sumon MH, Norton G, Brammer H, Islam R (2011) Organic matter - solid phase interactions are critical for predicting arsenic release and plant uptake in Bangladesh paddy soils. Environ Sci Technol 45:6080–6087
Xu XY, McGrath SP, Meharg AA, Zhao FJ (2008) Growing rice aerobically markedly decreases arsenic accumulation. Environ Sci Technol 42:5574–5579
Yang YP, Zhang HM, Yuan HY, Duan GL, Jin DC, Zhao FJ, Zhu YG (2018) Microbe mediated arsenic release from iron minerals and arsenic methylation in rhizosphere controls arsenic fate in soil-rice system after straw incorporation. Environ Pollut 236:598–608
Zhao FJ, Harris E, Yan J, Ma J, Wu L, Liu W, McGrath SP, Zhou J, Zhu YG (2013a) Arsenic methylation in soils and its relationship with microbial arsM abundance and diversity, and As speciation in rice. Environ Sci Technol 47:7147–7154
Zhao FJ, Zhu YG, Meharg AA (2013b) Methylated arsenic species in rice: geographic variation, origin and uptake mechanisms. Environ Sci Technol 47:3957–3966
Acknowledgements
NERC are kindly thanked for funding this work under the auspices of their Newton Critical Zone project. MB was funded through a commonwealth Scholarship. Sequencing was performed at the Genomics unit at Queen’s University Belfast.
Funding
Natural Environment Research Council,NE/N007417/1,Stephen Banward,NE/S009108/1,Stephen Banward,NE/S009124/1,Stephen Banward
Author information
Authors and Affiliations
Contributions
MB and EY conducted the microcosm work; MB and LL contributed equally to the writing of the manuscript. MB conducted the statistical analysis of the time-course chemical data and relevant writing, LL conducted the bioinformatics and statistical analysis of the time-course microbial data and integration of microbial and chemical time-course data and relevant writing; MC assisted in chemical analysis and contributed to the draft revisions; CM and AAM supervised the microcosm study, contributed to writing and revised the manuscript; GL collected the soil, and contributed to the draft revisions; YGZ and SB lead the CZ programme, helped conceptualise the project, and contributed to the draft revisions.
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no competing interests.
Ethicsal Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Begum, M., Li, L., Young, E. et al. Fertilization Enhances Grain Inorganic Arsenic Assimilation in Rice. Expo Health 16, 417–430 (2024). https://doi.org/10.1007/s12403-023-00563-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12403-023-00563-y