Abstract
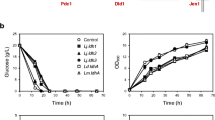
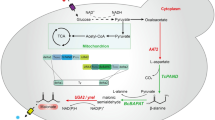
Microbial engineering based on synthetic biology can facilitate large-scale production of target products. In this study, the introduction of lactate dehydrogenase (LDH) enabled Saccharomyces cerevisiae to acquire the capacity for L-lactic acid (LA) production and the NADH/NAD+ ratio from 0.228 to 0.156, while the subsequent modification of carbon metabolism pathway led to a rapid increase of NADH/NAD+ even up to 0.337. By testing the effectiveness of four different redox systems, we demonstrated that dynamic regulation of additional redox genes to consume excessive NADH is more beneficial for LA accumulation, alleviating the negative effects of metabolic modification on hosts, and altering the distribution of metabolic flow. We first reported expression of GLT1 which coding glutamate synthase has the strongest ability to increase LA production and reduce NADH/NAD+. Combining metabolic engineering and cofactor engineering, the LA yield reached from 0.04 g/g to 0.37 g/g in YNB medium. Subsequently, strain PK27 produced 37.94 g/L LA with production yield of 0.66 g/g in YPD medium. Finally, the results could provide a reference that the potential under poor nutrient culture conditions and the direction and intensity of regulation of intracellular NADH/NAD+ for LA accumulation.






Similar content being viewed by others
References
Baek, S.H., E.Y. Kwon, Y.H. Kim, and J.S. Hahn. 2016. Metabolic engineering and adaptive evolution for efficient production of D-lactic acid in Saccharomyces cerevisiae. Applied Microbiology and Biotechnology 100 (6): 2737–2748.
Baek, S.H., E.Y. Kwon, S.J. Bae, B.R. Cho, S.Y. Kim, and J.S. Hahn. 2017. Improvement of D-lactic acid production in Saccharomyces cerevisiae under acidic conditions by evolutionary and rational metabolic engineering. Biotechnology Journal 12 (10): 1700015.
Chen, X.L., S.B. Li, and L.M. Liu. 2014. Engineering redox balance through cofactor systems. Trends in Biotechnology 32 (6): 337–343.
Chen, R.B., S. Yang, L. Zhang, and Y.J.J. Zhou. 2020. Advanced strategies for production of natural products in yeast. iScience 23 (3): 100879.
Dequin, S., and P. Barre. 1994. Mixed lactic acid-alcoholic fermentation by Saccharomyces cerevisiae expressing the Lactobacillus casei L(+)-LDH. Nature Biotechnology 12 (2): 173–177.
Han, L., and B. Liang. 2018. New approaches to NAD(P)H regeneration in the biosynthesis systems. World Journal of Microbiology and Biotechnology 34 (10): 141–148.
Hohmann, S., and H. Cederberg. 1990. Autoregulation may control the expression of yeast pyruvate decarboxylase structural genes PDC1 and PDC5. European Journal of Biochemistry 188 (3): 615–621.
Ishida, N., S. Saitoh, T. Ohnishi, K. Tokuhiro, E. Nagamori, K. Kitamoto, and H. Takahashi. 2006. Metabolic engineering of Saccharomyces cerevisiae for efficient production of pure L-(+)-lactic acid. Applied Microbiology and Biotechnology 131 (1–3): 795–807.
Kim, Y.H., J.Y. Kang, J.Y. Gil, S.Y. Kim, K.K. Shin, H.A. Kang, J.Y. Kim, O. Kwon, and D.B. Oh. 2017. Abolishment of N-glycan mannosylphosphorylation in glyco-engineered Saccharomyces cerevisiae by double disruption of MNN4 and MNN14 genes. Applied Microbiology and Biotechnology 101 (7): 2979–2989.
Komesu, A., J.A.R. de Oliveira, L.H. da Silva Martins, M.R.W. Maciel, and R. Maciel Filho. 2017. Lactic acid production to purification: A review. BioResources 12 (2): 4364–4383.
Kong, Q.X., J.G. Gu, L.M. Cao, A.L. Zhang, X. Chen, and X.M. Zhao. 2006. Improved production of ethanol by deleting FPS1 and over-expressing GLT1 in Saccharomyces cerevisiae. Biotechnology Letters 28 (24): 2033–2038.
Kong, Q.X., A.L. Zhang, L.M. Cao, and X. Chen. 2007. Over-expressing GLT1 in a gpd2Δ mutant of Saccharomyces cerevisiae to improve ethanol production. Applied Microbiology and Biotechnology 75 (6): 1361–1366.
Kong, X., B. Zhang, Y. Hua, Y.L. Zhu, W.J. Li, D.M. Wang, and J. Hong. 2018. Efficient L-lactic acid production from corncob residue using metabolically engineered thermo-tolerant yeast. Bioresource Technology 273: 220–230.
Kozak, B.U., H.M. van Rossum, K.R. Benjamin, L. Wu, J.M. Daran, J.T. Pronk, and A.J. van Maris. 2014. Replacement of the Saccharomyces cerevisiae acetyl-CoA synthetases by alternative pathways for cytosolic acetyl-CoA synthesis. Metabolic Engineering 21: 46–59.
Lee, J.Y., C.D. Kang, S.H. Lee, Y.K. Park, and K.M. Cho. 2015. Engineering cellular redox balance in Saccharomyces cerevisiae for improved production of L-lactic acid. Biotechnology and Bioengineering 112 (4): 751–758.
Lee, J.J., N. Crook, J. Sun, and H.S. Alper. 2016. Improvement of lactic acid production in Saccharomyces cerevisiae by a deletion of ssb1. Journal of Industrial Microbiology and Biotechnology 43 (1): 87–96.
Li, C.G., Q.Z. Zeng, M.Y. Chen, L.H. Xu, C.C. Zhang, F.Y. Mai, C.Y. Zeng, X.H. He, and D.Y. Ouyang. 2019. Evodiamine augments NLRP3 inflammasome activation and anti-bacterial responses through inducing alpha-tubulin acetylation. Frontiers in Pharmacology 10: 290.
Liao, Z.P., X.T. Yang, H.X. Fu, and J.F. Wang. 2019. The significance of aspartate on NAD(H) biosynthesis and ABE fermentation in Clostridium acetobutylicum ATCC 824. AMB Express 9 (1): 142.
Liu, J., H. Li, G. Zhao, Q. Caiyin, and J. Qiao. 2018. Redox cofactor engineering in industrial microorganisms: Strategies, recent applications and future directions. Journal of Industrial Microbiology and Biotechnology 45 (5): 313–327.
Ljungdahl, P.O., and B. Daignan-Fornier. 2012. Regulation of amino acid, nucleotide, and phosphate metabolism in Saccharomyces cerevisiae. Genetics 190 (3): 885–929.
Naghshbandi, M.P., M. Tabatabaei, M. Aghbashlo, V.K. Gupta, A. Sulaiman, K. Karimi, H. Moghimi, and M. Maleki. 2019. Progress toward improving ethanol production through decreased glycerol generation in Saccharomyces cerevisiae by metabolic and genetic engineering approaches. Renewable and Sustainable Energy Reviews 115: 109353.
Negoro, H., K. Matsumura, F. Matsuda, H. Shimizu, Y. Hata, and H. Ishida. 2020. Effects of mutations of GID protein-coding genes on malate production and enzyme expression profiles in Saccharomyces cerevisiae. Applied Microbiology and Biotechnology 104 (11): 4971–4983.
Nissen, T.L., M.C. Kielland-Brandt, J. Nielsen, and J. Villadsen. 2000. Optimization of ethanol production in Saccharomyces cerevisiae by metabolic engineering of the ammonium assimilation. Metabolic Engineering 2 (1): 69–77.
Pacheco, A., G. Talaia, J. Sa-Pessoa, D. Bessa, M.J. Goncalves, R. Moreira, S. Paiva, M. Casal, and O. Queiros. 2012. Lactic acid production in Saccharomyces cerevisiae is modulated by expression of the monocarboxylate transporters Jen1 and Ady2. FEMS Yeast Research 12 (3): 375–381.
Pimtong, V., S. Ounaeb, S. Thitiprasert, V. Tolieng, S. Sooksai, R. Boonsombat, S. Tanasupawat, S. Assabumrungrat, and N. Thongchul. 2017. Enhanced effectiveness of Rhizopus oryzae by immobilization in a static bed fermentor for L-lactic acid production. Process Biochemistry 52: 44–52.
Rabinowitz, J.D., and S. Enerback. 2020. Lactate: The ugly duckling of energy metabolism. Nature Metabolism 2 (7): 566–571.
Rigoulet, M., H. Aguilaniu, N. Averet, O. Bunoust, N. Camougrand, X. Grandier-Vazeille, C. Larsson, I.L. Pahlman, S. Manon, and L. Gustafsson. 2004. Organization and regulation of the cytosolic NADH metabolism in the yeast Saccharomyces cerevisiae. Molecular and Cellular Biochemistry 256 (1–2): 73–81.
Saitoh, S., N. Ishida, T. Onishi, K. Tokuhiro, E. Nagamori, K. Kitamoto, and H. Takahashi. 2005. Genetically engineered wine yeast produces a high concentration of L-lactic acid of extremely high optical purity. Applied Microbiology and Biotechnology 71 (5): 2789–2792.
Shi, L., and B.P. Tu. 2013. Acetyl-CoA induces transcription of the key G1 cyclin CLN3 to promote entry into the cell division cycle in Saccharomyces cerevisiae. Proceedings of the National Academy of Sciences of the United States of America 110 (18): 7318–7323.
Shu, C., C. Guo, S. Luo, S. Jiang, and Z. Zheng. 2015. Influence of altered NADH metabolic pathway on the respiratory-deficient mutant of Rhizopus oryzae and its L-lactate production. Applied Biochemistry and Biotechnology 176 (7): 2053–2064.
Siakeng, R., M. Jawaid, H. Ariffin, S.M. Sapuan, M. Asim, and N. Saba. 2018. Natural fiber reinforced polylactic acid composites: A review. Polymer Composites 40 (2): 446–463.
Singhvi, M., T. Zendo, and K. Sonomoto. 2018. Free lactic acid production under acidic conditions by lactic acid bacteria strains: Challenges and future prospects. Applied Microbiology and Biotechnology 102 (14): 5911–5924.
Song, J.Y., J.S. Park, C.D. Kang, H.Y. Cho, D. Yang, S. Lee, and K.M. Cho. 2016. Introduction of a bacterial acetyl-CoA synthesis pathway improves lactic acid production in Saccharomyces cerevisiae. Metabolic Engineering 35: 38–45.
Steffan, J.S., and L. McAlister-Henn. 1992. Isolation and characterization of the yeast gene encoding the MDH3 isozyme of malate dehydrogenase. Journal of Biological Chemistry 267 (34): 24708–24715.
Swamy, K.B.S., and N. Zhou. 2019. Experimental evolution: Its principles and applications in developing stress-tolerant yeasts. Applied Microbiology and Biotechnology 103 (5): 2067–2077.
Taskila, S., and H. Ojamo. 2013. The current status and future expectations in industrial production of lactic acid by lactic acid bacteria. In Lactic acid bacteria - R and D for food, health and livestock purposes, ed. M. Kongo, 615–632. London: IntechOpen.
Tian, X.J., A.L. Jiang, Y.Q. Mao, B. Wu, M.X. He, W. Hu, J.H. Chen, and W.J. Li. 2019. Efficient L-lactic acid production from purified sweet sorghum juice coupled with soybean hydrolysate as nitrogen source by Lactobacillus thermophilus A69 strain. Journal of Chemical Technology and Biotechnology 94 (6): 1752–1759.
Tokuhiro, K., N. Ishida, E. Nagamori, S. Saitoh, T. Onishi, A. Kondo, and H. Takahashi. 2009. Double mutation of the PDC1 and ADH1 genes improves lactate production in the yeast Saccharomyces cerevisiae expressing the bovine lactate dehydrogenase gene. Applied Microbiology and Biotechnology 82 (5): 883–890.
Tsuge, Y., N. Kato, S. Yamamoto, M. Suda, T. Jojima, and M. Inui. 2019. Metabolic engineering of Corynebacterium glutamicum for hyperproduction of polymer-grade L- and D-lactic acid. Applied Microbiology and Biotechnology 103 (8): 3381–3391.
Turner, T.L., S. Lane, L.N. Jayakody, G.C. Zhang, H. Kim, W. Cho, and Y.S. Jin. 2019. Deletion of JEN1 and ADY2 reduces lactic acid yield from an engineered Saccharomyces cerevisiae, in xylose medium, expressing a heterologous lactate dehydrogenase. FEMS Yeast Research 19 (6): 19.
Upadhyaya, B.P., L.C. DeVeaux, and L.P. Christopher. 2014. Metabolic engineering as a tool for enhanced lactic acid production. Trends in Biotechnology 32 (12): 637–644.
Vemuri, G.N., M.A. Eiteman, J.E. McEwen, L. Olsson, and J. Nielsen. 2007. Increasing NADH oxidation reduces overflow metabolism in Saccharomyces cerevisiae. Proceedings of the National Academy of Sciences of the United States of America 104 (7): 2402–2407.
Wang, Z.K., C.J. Gao, Q. Wang, Q.F. Liang, and Q.S. Qi. 2012. Production of pyruvate in Saccharomyces cerevisiae through adaptive evolution and rational cofactor metabolic engineering. Biochemical Engineering Journal 67: 126–131.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (31460026), the Natural Science Foundation of Guangxi Province (2018GXNSFAA050126, 2020GXNSFAA297104), the Young & Middle-aged Backbone Teachers Training Project in Colleges and Universities of Guangxi province, the China Agriculture Research System of MOF and MARA, and the Science and technology project of Chongzuo (FA2020001).
Author information
Authors and Affiliations
Contributions
Fuxiao Li and Xin Wei conceived the study. Fuxiao Li and Qinju Sun performed all the experiments and original draft investigation. Yan Guo performed part of the analytical method. Jidong Liu did methodology validation, supervised the experiments, prepared, review, and edited the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Human and Animal Participants
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, F., Wei, X., Sun, Q. et al. Production of L-Lactic Acid in Saccharomyces cerevisiae Through Metabolic Engineering and Rational Cofactor Engineering. Sugar Tech 24, 1272–1283 (2022). https://doi.org/10.1007/s12355-022-01142-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12355-022-01142-2