Abstract
Alpha-fetoprotein (AFP)-producing adenocarcinoma is known for its rapid progression and poor prognosis, and chemotherapy regimens are yet to be standardized. Here we describe the first report of AFP-producing adenocarcinoma with calcification. The metastatic route was visualized from the calcification, and combination chemotherapy was performed. A 77-year-old Japanese man was transferred to our hospital for treatment of liver tumors. Computed tomography (CT) revealed multiple liver tumors with portal vein tumor thrombosis. The tumors were highly attenuated before enhancement, suggesting various degrees of calcification. Serum levels of carcinoembryonic antigen (CEA), AFP, and the proportion of fucosylated AFP were considerably elevated. Gastroduodenoscopy revealed an elevated tumor occupying the duodenal bulb with an ulcerative lesion in the vicinity of the gastroduodenal junction, and biopsy specimens from the duodenum and liver revealed medullary adenocarcinoma with calcification. Three-dimensional reconstruction of CT images clearly showed that the calcified lesions had spread from the gastroduodenal junction to the liver via a route comprising the corresponding local vein, the superior mesenteric vein, and portal vein. The patient was accordingly diagnosed with calcified AFP-producing adenocarcinoma with multiple liver metastases. Combination chemotherapy using TS-1 and cisplatin (CDDP) resulted in a striking response for the initial 4 months in terms of tumor markers and CT findings. This is the first report of AFP-producing adenocarcinoma with calcification. A metastatic route from the primary tumor to the liver was clearly visualized by tracing the calcified lesions. Combination chemotherapy based on 5-fluorouracil and CDDP may have the potential to prolong survival.
Similar content being viewed by others
Introduction
Alpha-fetoprotein (AFP) was originally identified from fetal tissue and is normally produced in the fetal liver and yolk sac [1]. It is also upregulated during liver regeneration and is a well-known tumor marker for hepatocellular carcinoma [2]. Furthermore, in studies using embryonic stem (ES) cells, AFP is often used as a representative endoderm marker [3]. AFP-producing cancers other than hepatocellular carcinoma (HCC) are often reported, including those of the stomach [4], duodenum [5], papilla of Vater [6] and pancreas [7, 8]. The most common of these is AFP-producing gastric cancer [9], which has a reported frequency of 1.5–15% of all gastric cancers [10]. The clinical course of AFP-producing adenocarcinoma is familiar with its rapid progression and poor prognosis. Chang et al. [11] reported that a 5-year survival rate of AFP-producing gastric cancer is only 8.3%. AFP-producing gastric cancer also reportedly has weak apoptotic activity and high neovascularization potential compared with its AFP-negative counterpart [12]; however, to the best of our knowledge, AFP-producing adenocarcinoma with calcification has not yet been reported.
On the other hand, although there is no standard chemotherapy for this rapidly progressive advanced AFP-producing adenocarcinoma, various chemotherapy regimens for AFP-producing gastric cancer have been frequently reported. Favorable results have been observed with combination chemotherapies, particularly those using cisplatin (CDDP) and 5-fluorouracil (5-FU) as key drugs [13–19].
In this case history, we report a case of AFP-producing adenocarcinoma with calcification that was derived from the duodenal bulb or the gastroduodenal junction. This tumor showed calcification and had hematogenously metastasized to the liver. This metastasis route was surprising and could be traced by the calcification. We performed computed tomography arterioportal angiography (CTAP) and CT during hepatic angiography (CTA), and examined pathological tissue from the primary tumor at the gastroduodenal junction and the liver metastasis. Combination chemotherapy using TS-1 and CDDP was performed as first-line chemotherapy and the tumor initially responded favorably to this before toxicity developed.
Case report
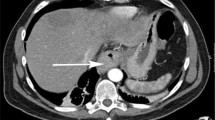
A 77-year-old man was transferred to Niigata University hospital for evaluation and treatment of multiple liver tumors with calcification. He was a heavy drinker and had previously been diagnosed with chronic alcoholic liver disease. Although hepatitis virus markers of HBsAg and anti-HCV were negative, anti-HBs antibody was positive. Laboratory data on admission revealed the elevation of liver and biliary enzymes as follows: aspartate aminotransferase (AST), 64 IU/l (normal 13–33 IU/l); alanine aminotransferase (ALT), 45 IU/l (normal 8–42 IU/l); alkaline phosphatase (ALP), 467 IU/l (normal 115–359 IU/l); and γ-glutamyltranspeptidase (γ-GTP), 270 IU/l (normal 10–47 IU/l). Other laboratory data relating to chemotherapy were as follows; total bilirubin (TB), 0.9 mg/dl (normal 0.3–1.1 mg/dl); white blood cell count, 6570/mm3 (normal 3590–9640/mm3); platelet count, 9.9 × 104/mm3 (normal 14.8 × 104–33.9 × 104/mm3). The tumor marker AFP, the proportion of fucosylated AFP, and carcinoembryonic antigen (CEA) were elevated at 3190 ng/ml (normal <6 ng/ml), 93.1% (normal <10%), and 72.2 ng/ml (normal 0.7–4.2 ng/ml), respectively. Abdominal CT on admission revealed multiple liver tumors with various degrees of calcification and portal vein tumor thrombosis with calcification. Furthermore, duodenal wall thickening with low-level calcification was detected. To distinguish between primary and metastatic liver tumor, CTAP, gastroduodenoscopy, 3-dimensional (3D) CT, and histopathological examination were performed. Liver tumors could be detected as CT defects during CTAP, were hypervascular in the first phase of dual-phase CTA, and showed corona-like enhancement in the second phase of dual-phase CTA (Fig. 1). Gastroduodenoscopy using a side-viewing scope revealed that an elevated tumor occupied the duodenal bulb with small ulcerative lesions in the vicinity of the gastroduodenal junction (Fig. 2a–c). 3D CT showed the extent of calcification and indicated a metastasis route from the duodenum to the liver, from the corresponding vein near the duodenum to the superior mesenteric vein to the portal vein (Fig. 2d, f). Histopathological analysis of biopsy specimens from the duodenal and liver tumors revealed medullary adenocarcinoma rather than hepatocellular carcinoma. Calcification was detected both in the duodenal tumor and liver tumors. Furthermore, immunohistochemical analysis revealed that specimens from the duodenal tumor were positive for CEA and AFP, and those from the liver tumors were weakly positive for Hep-Per1 and AFP and strongly positive for Muc1 (Fig. 3). Based on these results, we diagnosed AFP-producing adenocarcinoma arising from the duodenal bulb or gastroduodenal junction with metastasis to the liver.
Plain computed tomography (CT), CT arterioportal angiography (CTAP) and CT during hepatic angiography (CTA). Plain CT revealed tumors with calcification in the liver. Calcification was also detected in the portal vein and duodenum (a, e). CTAP revealed many defects in the liver and one in the portal vein (b, f). The first phase of dual-phase CTA revealed many hypervascular tumors, which were consistent with CTAP defects (c, g). These hypervascular tumors were detected as corona-like enhancements in second phase of dual-phase CTA (d, h). White arrows indicate the portal vein tumor thrombosis
Gastroduodenoscopy (a–c) and three-dimensional (3D) CT (d, f). Gastroduodenoscopy revealed that the oral edge of the tumor was located at the gastroduodenal junction where a small ulcer was detected and sequentially the elevated tumor occupied the duodenal bulb (a–c). 3D CT analysis revealed the metastatic route, which was apparent because of calcification (d, f a–c indicate the horizontal section of each CT level. Dotted circles mark the calcification). The calcification can be traced from the duodenum (1), to a vein near the duodenum (2), to the superior mesenteric vein (3) and portal vein (4) (f)
Histopathological examination. Histopathological appearance of the tumors is shown (a–d, the gastroduodenal tumor; e–j, the liver tumors). Both the gastroduodenal tumor and liver tumors are medullary adenocarcinomas (a, b, e–f). The gastroduodenal tumor was positive for carcinoembryonic antigen (CEA) (c) and alpha-fetoprotein (AFP) (d). The liver tumors containing considerable calcification (e–f), were weakly positive for AFP (h) and Hep-per1 (i), and strongly positive for Muc1 (j)
Since there is no standard therapy for AFP-producing adenocarcinoma, chemotherapy according to the regimen for gastric cancer was selected for this patient after obtaining informed consent. Initially, combination chemotherapy with TS-1 and CDDP was repeated every 5 weeks. TS-1 was administered orally (65 mg/m2/day) for 3 weeks and then ceased for 2 weeks. At the same time, 20 mg/m2 CDDP was administered intravenously on 2 days; day 1 and day 8 (on day 1 of the first course, this was substituted for 60 mg/m2 of IA-call® administered intra-arterially from the proper hepatic artery). After the first course of TS-1 and CDDP chemotherapy, AFP and CEA decreased rapidly (Fig. 4a, b), and CT revealed shrinkage of the tumors in the liver and portal vein (Fig. 4c–h). We concluded that partial response could be achieved by this chemotherapy regimen. Although tumors in the liver and portal vein had reduced in size, calcification of these sites remained. After the 4th course of TS-1 and CDDP, tumor markers increased again, and the chemotherapy regimen was changed to TS-1 and paclitaxel and then to CPT-11 (irinotecan) and CDDP. However, adverse events during the therapy (grade 3 leucopenia and grade 3 thrombocytopenia according to Common Terminology Criteria for Adverse Events Version 4.0) developed and precluded sufficient chemotherapy administration; thus, favorable results could not be obtained. The patient died approximately 1 year after the detection of multiple liver tumors.
Evaluation of chemotherapy in terms of tumor markers and CT findings. After the first course of chemotherapy, serum levels of the tumor markers AFP and CEA decreased rapidly; however, serum levels of these markers increased gradually thereafter (a, b). CT findings before chemotherapy (c–e) and after chemotherapy (f–h) are also shown. After the first course of chemotherapy, CT revealed the disappearance or shrinkage of many hypervascular tumors (d, g). The portal vein tumor thrombosis also decreased in size (e, h); however, calcification itself was unchanged (c, f). Black arrows indicate the portal vein tumor thrombosis
Discussion
We have reported here what appears to be the first case of AFP-producing adenocarcinoma with calcification. This tumor formed a small ulcer at the gastroduodenal junction; however, the main tumor site was the duodenum. Since AFP-producing adenocarcinoma from duodenum is very rare, we considered the pathophysiological aspects of and therapy for this tumor in comparison to those of previously reported AFP-producing gastric cancer. AFP-producing gastric cancer can be divided into three types: (1) hepatoid type, (2) yolk-sac tumor-like type, and (3) fetal gastrointestinal type [20]. The present case exhibited hepatoid differentiation and AFP production; therefore, we diagnosed hepatoid-type medullary cancer. The most remarkable aspect of this case was that the tumors had different degrees of calcification. Until 1994, 56 cases of gastric cancer with calcification had been reported in the Japanese literature [21]. A review of these suggests three possible mechanisms of calcification: (1) dystrophic calcification of necrotic or degenerative tissue; (2) ontogenic calcification due to glycoprotein in the mucin component of the tumor; and (3) metastatic calcification caused by hypercalcemia. In the present case, the tumors progressed rapidly, and mucin and hypercalcemia were not detected; thus, dystrophic calcification of necrotic or degenerative tissue seems reasonable. In general, hematogenous metastasis, lymphogenous metastasis [22] and peritoneal dissemination [23] are well known as metastatic routes of gastric cancer. This case clearly showed hematogenous metastasis to the liver by calcification. CT revealed the different levels of calcification among the metastatic tumors; however, every tumor that was examined pathologically included different levels of calcification and the tumors themselves had common features of metastatic liver tumors.
In the present case, the proportion of fucosylated AFP was extremely high. Our department previously reported that metastatic liver tumors showed very high proportions of fucosylated AFP (76 ± 25% compared with 42 ± 30% for HCC) [24]. The fucosylation index of AFP in this case remained over 90%. This supports our previous findings and indicates that the fucosylation index of AFP could be one way to distinguish between HCC and AFP-producing adenocarcinoma.
AFP-producing gastric cancer is known for its high proliferative ability and poor prognosis. Recent reports have described that Ki-67, hepatocyte growth factor (HGF) and its receptor c-Met, and vascular endothelial growth factor (VEGF) and its isoform VEGF-C, were highly expressed by this tumor and were related to malignant potential [12, 25–27]. Although AFP-producing gastric cancer is known for its poor prognosis, effective chemotherapy regimens have been described for this tumor. These regimens have included TS-1, CDDP, and paclitaxel [19]; paclitaxel and TS-1 or 5-FU [18]; CPT-11 plus low-dose CDDP (for AFP-producing gastric cancer with multiple liver metastasis) [16]; and epirubicin (EPI), 5-FU, and leucovorin (LV) (as pre-operative combination chemotherapy) [13]. Moreover, Kochi et al. [14] reported combination chemotherapy with 5-FU, LV, etoposide, and CDDP (FLEP therapy) for inoperable stage IV gastric cancer and also concluded that FLEP chemotherapy was more effective for stage IV AFP-producing gastric cancer than for stage IV non-AFP-producing gastric cancer. These reports suggest that CDDP and 5-FU or TS-1 could be key drugs to combat AFP-producing adenocarcinoma, and as TS-1 plus CDDP is well known as an effective therapy for gastric cancer [28], we therefore used a combination of CDDP and TS-1 as first-line chemotherapy. The patient responded very well to this, but he developed hematological toxicity including leucopenia and thrombocytopenia, which prevented him continuing as scheduled from the end of first-line chemotherapy. After first-line treatment, we attempted to use other anti-cancer drugs such as paclitaxel and CPT-11, but ongoing hematological toxicity precluded sufficient chemotherapy. Nonetheless, the present combination chemotherapy allowed the patient to survive for 1 year while maintaining quality of life, despite the multiple liver metastases with portal vein tumor thrombosis. Hence, combination chemotherapy based on CDDP and 5-FU should be considered for such patients.
For the first course of first-line chemotherapy for this patient, we gave intra-arterial IA-call® instead of intravenous CDDP. Shiochi et al. [17] reported that the combination of systemic chemotherapy, transcatheter arterial embolization, and hepatic infusion chemotherapy was effective for AFP-producing gastric cancer and that the patient survived for more than 3 years. Shoda et al. [29] reported successful treatment of spontaneous rupture of metastatic AFP-positive gastric cancer of the liver by transarterial embolization. AFP-producing gastric cancers are known to be hypervascular; thus, transarterial chemotherapy may be an attractive method for the treatment of liver metastases of AFP-producing adenocarcinoma.
References
Gitlin D, Perricelli A, Gitlin GM. Synthesis of alpha-fetoprotein by liver, yolk sac, and gastrointestinal tract of the human conceptus. Cancer Res. 1972;32:979–82.
Purves LR, Bersohn I, Geddes EW. Serum alpha-feto-protein and primary cancer of the liver in man. Cancer. 1970;25:1261–70.
Umeda K, Heike T, Yoshimoto M, Shiota M, Suemori H, Luo HY, et al. Development of primitive and definitive hematopoiesis from nonhuman primate embryonic stem cells in vitro. Development. 2004;131:1869–79.
Bourreille J, Metayer P, Sauger F, Matray F, Fondimare A. Existence of alpha feto protein during gastric-origin secondary cancer of the liver. Presse Med. 1970;78:1277–8.
Takashima H, Kimura H, Nakamura H, Myojo S, Okuyama Y, Sugeta N, et al. A case of AFP producing endocrine cell carcinoma of the duodenum. Nippon Shokakibyo Gakkai Zasshi. 2002;99:798–802.
Gardiner GW, Lajoie G, Keith R. Hepatoid adenocarcinoma of the papilla of Vater. Histopathology. 1992;20:541–4.
Iwai K, Ishikura H, Inoue T, Yoshiki T. A case of AFP-positive pancreas papillary carcinoma suggestive of a primitive endoderm phenotype. Acta Pathol Jpn. 1993;43:434–9.
Tanno S, Obara T, Fujii T, Izawa T, Mizukami Y, Saitoh Y, et al. alpha-Fetoprotein-producing adenocarcinoma of the pancreas presenting focal hepatoid differentiation. Int J Pancreatol. 1999;26:43–7.
Alpert E, Pinn VW, Isselbacher KJ. Alpha-fetoprotein in a patient with gastric carcinoma metastatic to the liver. N Engl J Med. 1971;285:1058–9.
Inagawa S, Shimazaki J, Hori M, Yoshimi F, Adachi S, Kawamoto T, et al. Hepatoid adenocarcinoma of the stomach. Gastric Cancer. 2001;4:43–52.
Chang YC, Nagasue N, Kohno H, Taniura H, Uchida M, Yamanoi A, et al. Clinicopathologic features and long-term results of alpha-fetoprotein-producing gastric cancer. Am J Gastroenterol. 1990;85:1480–5.
Koide N, Nishio A, Igarashi J, Kajikawa S, Adachi W, Amano J. Alpha-fetoprotein-producing gastric cancer: histochemical analysis of cell proliferation, apoptosis, and angiogenesis. Am J Gastroenterol. 1999;94:1658–63.
Ihling C, Schaefer HE, Baumgartner U, Riede UN. Hepatoid adenocarcinoma of the stomach: a case report. Gen Diagn Pathol. 1995;141:61–5.
Kochi M, Fujii M, Kaiga T, Takahashi T, Morishita Y, Kobayashi M, et al. FLEP chemotherapy for alpha-fetoprotein-producing gastric cancer. Oncology. 2004;66:445–9.
Nakajima T, Ota K, Ishihara S, Oyama S, Nishi M, Ohashi Y, et al. Combined intensive chemotherapy and radical surgery for incurable gastric cancer. Ann Surg Oncol. 1997;4:203–8.
Shimada S, Hayashi N, Marutsuka T, Baba Y, Yokoyama S, Iyama K, et al. Irinotecan plus low-dose cisplatin for alpha-fetoprotein-producing gastric carcinoma with multiple liver metastases: report of two cases. Surg Today. 2002;32:1075–80.
Shiochi H, Yamada M, Kishina M, Murawaki Y, Miura M, Azumi T, et al. A case of AFP-producing gastric cancer responding to the combination of systemic chemotherapy, transcatheter arterial embolization and hepatic infusion chemotherapy. Gan To Kagaku Ryoho. 2009;36:843–6.
Takeyama H, Sawai H, Wakasugi T, Takahashi H, Matsuo Y, Ochi N, et al. Successful paclitaxel-based chemotherapy for an alpha-fetoprotein-producing gastric cancer patient with multiple liver metastases. World J Surg Oncol. 2007;5:79.
Usuba T, Toyama Y, Watanabe K, Kashiwagi H, Yanaga K. Combination chemotherapy using TS-1, paclitaxel and cisplatin for multiple lung metastases from AFP-producing gastric cancer: a case report. Hepatogastroenterology. 2007;54:1302–4.
Motoyama T, Aizawa K, Watanabe H, Fukase M, Saito K. Alpha-fetoprotein producing gastric carcinomas: a comparative study of three different subtypes. Acta Pathol Jpn. 1993;43:654–61.
Ichiishi E, Kogawa T, Takeda S, Yanagida K, Yoshikawa T, Kondo M. Eight cases of gastric tumors with calcification. Intern Med. 1995;34:1038–42.
Michelassi F, Takanishi DM Jr, Pantalone D, Hart J, Chappell R, Block GE. Analysis of clinicopathologic prognostic features in patients with gastric adenocarcinoma. Surgery. 1994;116:804–9. discussion 809–10.
Glockzin G, Schlitt HJ, Piso P. Peritoneal carcinomatosis: patients selection, perioperative complications and quality of life related to cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. World J Surg Oncol. 2009;7:5.
Aoyagi Y, Suzuki Y, Igarashi K, Saitoh A, Oguro M, Yokota T, et al. The usefulness of simultaneous determinations of glucosaminylation and fucosylation indices of alpha-fetoprotein in the differential diagnosis of neoplastic diseases of the liver. Cancer. 1991;67:2390–4.
Amemiya H, Kono K, Mori Y, Takahashi A, Ichihara F, Iizuka H, et al. High frequency of c-Met expression in gastric cancers producing alpha-fetoprotein. Oncology. 2000;59:145–51.
Kamei S, Kono K, Amemiya H, Takahashi A, Sugai H, Ichihara F, et al. Evaluation of VEGF and VEGF-C expression in gastric cancer cells producing alpha-fetoprotein. J Gastroenterol. 2003;38:540–7.
Kono K, Amemiya H, Sekikawa T, Iizuka H, Takahashi A, Fujii H, et al. Clinicopathologic features of gastric cancers producing alpha-fetoprotein. Dig Surg. 2002;19:359–65. discussion 365.
Koizumi W, Narahara H, Hara T, Takagane A, Akiya T, Takagi M, et al. S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet Oncol. 2008;9:215–21.
Sohda T, Hanano T, Miyamoto H, Kitano Y, Iwata K, Yokoyama M, et al. Spontaneous rupture of metastatic alpha-fetoprotein-producing gastric cancer of the liver. Hepatol Int. 2008;2:258–61.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tsuchiya, A., Kanefuji, T., Suda, T. et al. Alpha-fetoprotein-producing adenocarcinoma in which the metastatic route was determined from calcified lesions. Clin J Gastroenterol 4, 89–94 (2011). https://doi.org/10.1007/s12328-011-0209-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12328-011-0209-x