Abstract
Introduction
A trial-based assessment was completed to evaluate the cost-effectiveness of ceritinib as a first-line treatment for advanced non-small cell lung cancer (NSCLC) with rearrangement of anaplastic lymphoma kinase.
Methods
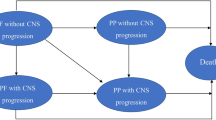
Based on the disease situation of advanced NSCLC, a Markov model was constructed to estimate the costs and benefits of ceritinib and platinum-based chemotherapy. The cost information and health utilities were obtained from published literature. The incremental cost-effectiveness ratio was calculated. The stability of the model was verified by sensitivity analyses.
Results
The base case analysis results indicated that compared with platinum-based chemotherapy, ceritinib therapy would increase benefits in a 5-, 10- and 15-year time horizon, with extra costs of $230,661.61, $149,321.52 and $136,414.43 per quality-adjusted life-year gained, respectively. The most sensitive parameter in the model analysis was the cost of ceritinib. Probabilistic sensitivity analysis suggested that at the current price of ceritinib, the chance of ceritinib being cost-effective was 0 at the willingness-to-pay threshold of $27,142.85 per quality-adjusted life-year (three times the per capita gross domestic product of China).
Conclusion
As a first-line treatment for advanced NSCLC with rearrangement of anaplastic lymphoma kinase, ceritinib is unlikely to be cost-effective at the current price from the Chinese healthcare perspective. To meet the treatment demands of patients, it may be a better option to reduce the price or provide appropriate drug assistance policies.





Similar content being viewed by others
References
Global Burden of Disease Cancer Collaboration, Fitzmaurice C, Allen C, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: a systematic analysis for the global burden of disease study. JAMA Oncol. 2017;3(4):524–48.
Zaidi Z, Hamdi Cherif M. PS01.07: Geographical distribution of lung cancer mortality worldwide: topic: pathology. J Thorac Oncol. 2016;11(11S):S273–4.
Wong MCS, Lao XQ, Ho KF, Goggins WB, Tse SLA. Incidence and mortality of lung cancer: global trends and association with socioeconomic status. Sci Rep. 2017;7(1):14300.
Chen W, Zheng R, Zhang S, et al. Cancer incidence and mortality in China in 2013: an analysis based on urbanization level. Chin J Cancer Res. 2017;29(1):1–10.
Zhang X, Liu S, Liu Y, et al. Economic burden for lung cancer survivors in urban China. Int J Environ Res Public Health. 2017;14(3):308.
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90.
Nation Comprehensive Cancer Network. Non-small cell lung cancer [version 2.2019]. https://www.nccn.org/. Accessed 13 Aug 2019.
Tan DS, Araujo A, Zhang J, et al. Comparative efficacy of ceritinib and crizotinib as initial ALK-targeted therapies in previously treated advanced NSCLC: an adjusted comparison with external controls. J Thorac Oncol. 2016;11(9):1550–7.
Amin AD, Li L, Rajan SS, et al. TKI sensitivity patterns of novel kinase-domain mutations suggest therapeutic opportunities for patients with resistant ALK+ tumors. Oncotarget. 2016;7(17):23715–29.
Zhu Q, Hu H, Weng DS, et al. Pooled safety analyses of ALK-TKI inhibitor in ALK-positive NSCLC. BMC Cancer. 2017;17(1):412.
Li S, Qi X, Huang Y, Liu D, Zhou F, Zhou C. Ceritinib (LDK378): a potent alternative to crizotinib for ALK-rearranged non-small-cell lung cancer. Clin Lung Cancer. 2015;16(2):86–91.
Soria JC, Tan DSW, Chiari R, et al. First-line ceritinib versus platinum-based chemotherapy in advanced ALK-rearranged non-small-cell lung cancer (ASCEND-4): a randomised, open-label, phase 3 study. Lancet. 2017;389(10072):917–29.
Wan X, Zhang Y, Tan C, et al. First-line nivolumab plus ipilimumab vs sunitinib for metastatic renal cell carcinoma: a cost-effectiveness analysis. JAMA Oncol. 2019;5(4):491–6.
Murray CJ, Evans DB, Acharya A, Baltussen RM. Development of WHO guidelines on generalized cost-effectiveness analysis. Health Econ. 2000;9(3):235–51.
China Center for Health Economic Research. China guidelines for pharmacoeconomic evaluations (Version 2011) [in Chinese] (2011). https://tools.ispor.org/peguidelines/. Accessed 13 Aug 2019.
The People’s Bank of China. Exchange rate. http://www.pbc.gov.cn. Accessed 13 Aug 2019.
Cho BC, Kim DW, Bearz A, et al. ASCEND-8: a randomized phase 1 study of ceritinib, 450 mg or 600 mg, taken with a low-fat meal versus 750 mg in fasted state in patients with anaplastic lymphoma kinase (ALK)-rearranged metastatic non-small cell lung cancer (NSCLC). J Thorac Oncol. 2017;12:1357–67.
Cho BC, Obermannova R, Bearz A, et al. Efficacy and safety of ceritinib (450 mg/d or 600 mg/d) with food versus 750-mg/d fasted in patients with ALK receptor tyrosine kinase (ALK)-positive NSCLC: primary efficacy results from the ASCEND-8 study. J Thorac Oncol. 2019;14:1255–65.
Novartis Pharmaceuticals Corporation. Zykadia dosing and administration. https://www.hcp.novartis.com/products/zykadia/alk-nsclc/dosing-administration. Accessed 13 Aug 2019.
European Medical Agency. Zykadia European public assessment report. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/003819/WC500187504.pdf. Accessed 13 Aug 2019.
Zeng X, Karnon J, Wang S, et al. The cost of treating advanced non-small cell lung cancer: estimates from the Chinese experience. PLoS One. 2012;7(10):e48323.
Zeng X, Peng L, Li J, et al. Cost-effectiveness of continuation maintenance pemetrexed after cisplatin and pemetrexed chemotherapy for advanced nonsquamous non-small-cell lung cancer: estimates from the perspective of the Chinese health care system. Clin Ther. 2013;35(1):54–65.
Hurry M, Zhou ZY, Zhang J, et al. Cost-effectiveness of ceritinib in patients previously treated with crizotinib in anaplastic lymphoma kinase positive (ALK+) non-small cell lung cancer in Canada. J Med Econ. 2016;19(10):936–44.
Lu S, Ye M, Ding L, Tan F, Fu J, Wu B. Cost-effectiveness of gefitinib, icotinib, and pemetrexed-based chemotherapy as first-line treatments for advanced non-small cell lung cancer in China. Oncotarget. 2017;8(6):9996–10006.
Chongqing T, Liubao P, Xiaohui Z, et al. Cost-utility analysis of the newly recommended adjuvant chemotherapy for resectable gastric cancer patients in the 2011 Chinese National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology: Gastric Cancer. Pharmacoeconomics. 2014;32(3):235–43.
Wan XM, Peng LB, Ma JA, Li YJ. Economic evaluation of nivolumab as a second-line treatment for advanced renal cell carcinoma from US and Chinese perspectives. Cancer. 2017;123(14):2634–41.
Chouaid C, Agulnik J, Goker E, et al. Health-related quality of life and utility in patients with advanced non-small-cell lung cancer: a prospective cross-sectional patient survey in a real-world setting. J Thorac Oncol. 2013;8(8):997–1003.
Novello S, Barlesi F, Califano R, et al. Metastatic non-small-cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27(Suppl 5):v1–27.
Barron A, Wilsdon T. Challenging perceptions about oncology product pricing in breast and colorectal cancer. Pharmaceut Med. 2016;30(6):321–6.
Migliorino MR, Santo A, Romano G, et al. Economic burden of patients affected by non-small cell lung cancer (NSCLC): the LIFE study. J Cancer Res Clin Oncol. 2017;143(5):783–91.
Ocana A, Amir E, Tannock IF. Toward value-based pricing to boost cancer research and innovation. Cancer Res. 2016;76(11):3127–9.
Salmasi S, Lee KS, Ming LC, et al. Pricing appraisal of anti-cancer drugs in the south east Asian, western Pacific and east Mediterranean region. BMC Cancer. 2017;17(1):903.
Balu S, Cerezo-Camacho O, Smith NJ, Beckerman R. Cost-effectiveness of ceritinib versus current therapies for chemotherapy-experienced anaplastic lymphoma kinase positive non-small cell lung cancer patients in Mexico. Value Health. 2015;18(7):A821.
Zhou Z, Zhang J, Fan L, et al. Cost-effectiveness of ceritinib in the treatment of previously treated anaplastic lymphoma kinase-positive (ALK+) non-small cell lung cancer in the United Kingdom. Value Health. 2015;18(7):A455–6.
Zhou ZY, Mutebi A, Han S, et al. Cost-effectiveness of ceritinib in previously untreated anaplastic lymphoma kinase-positive metastatic non-small cell lung cancer in the United States. J Med Econ. 2018;21(6):577–86.
National Bureau of Statistics of China. China statistical year book 2018. http://www.stats.gov.cn/tjsj/ndsj/2018/indexeh.htm. Accessed 13 Aug 2019.
Shi J, Yao Y, Liu G. Modeling individual health care expenditures in China: evidence to assist payment reform in public insurance. Health Econ. 2018;27:1945–62.
Gao SN, Liu GQ. The application of pharmacoeconomics in the field of medicine and health [in Chinese]. Drug Econ. 2017;12(8):16–8.
Peng J, Tan C, Zeng X, Liu S. Cost-effectiveness analysis of capecitabine monotherapy versus capecitabine plus oxaliplatin in elderly patients with advanced gastric cancer. PLoS One. 2018;13(6):e0199553.
Announcement of the “2019 National Medical Insurance Drug Catalogue Adjustment Work Plan”. China Health Insurance. https://www.zgylbx.com/. Accessed 13 Aug 2019.
Koh L, Glaetzer C, Chuen Li S, Zhang M. Health technology assessment, international reference pricing, and budget control tools from China’s perspective: what are the current developments and future considerations? Value Health Reg Issues. 2016;9:15–21.
Acknowledgements
Funding
This work and associated journal article processing charges were supported by the National Natural Science Foundation of China (grant numbers 81401547, 81603081); and the Key Science-Technology Research and Development Program of Hunan Province (grant number 2016JC2062). All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosures
Ye Peng, Fang Ma, Chongqing Tan, Xiaomin Wan, Lidan Yi, Liubao Peng and Xiaohui Zeng have nothing to disclose.
Compliance with Ethics Guidelines
Because our economic evaluation was based on a mathematical model to simulate the patient’s lifetime, it did not require the approval of the independent ethics committee. This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Enhanced Digital Features
To view enhanced digital features for this article go to https://doi.org/10.6084/m9.figshare.9873323.
Rights and permissions
About this article
Cite this article
Peng, Y., Ma, F., Tan, C. et al. Model-Based Economic Evaluation of Ceritinib and Platinum-Based Chemotherapy as First-Line Treatments for Advanced Non-Small Cell Lung Cancer in China. Adv Ther 36, 3047–3058 (2019). https://doi.org/10.1007/s12325-019-01103-4
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-019-01103-4