Abstract
Introduction
Aprepitant, a selective neurokinin-1 receptor antagonist approved for prevention of chemotherapy-induced nausea and vomiting (CINV), is an inhibitor of the cytochrome P450 3A4 (CYP3A4) enzyme, which is involved in the clearance of several chemotherapeutic agents. Here we evaluated the efficacy and toxicity of a combination of aprepitant, palonosetron, and dexamethasone as antiemetic prophylaxis in sarcoma patients receiving ifosfamide and doxorubicin chemotherapy, and examined the potential of aprepitant to affect the pharmacokinetics of ifosfamide, which is primarily metabolized by CYP3A4.
Methods
A total of 108 sarcoma patients were randomly assigned to either the aprepitant group (antiemetic regimen: aprepitant, palonosetron, and dexamethasone) or the control group (antiemetic regimen: palonosetron and dexamethasone). Data on nausea, vomiting, and use of rescue medication were collected, and the primary efficacy end point was the proportion of patients with complete response (CR), defined as no vomiting and no use of rescue therapy during 120 h after initiation of chemotherapy. Tolerability was evaluated on the basis of reported adverse events and laboratory assessments. Blood samples for ifosfamide pharmacokinetic analysis were collected in ten patients.
Results
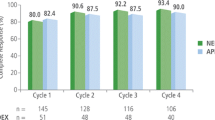
The percentage of patients achieving CR was significantly higher in the aprepitant group compared with that in the control group in the acute, delay, and overall phase (78.4% vs. 59.3%, 74.5% vs. 48.1%, and 68.6% vs. 37.0%, p < 0.05, respectively). No significant differences of adverse events or hematological toxicity were detected between the two groups. Concomitant administration of aprepitant did not cause any statistically significant changes in ifosfamide pharmacokinetics. Values for aprepitant group vs. control group were as follows: geometric mean of Cmax was 119 vs. 120 ng/mL, AUC0–last was 648 vs. 635 ng h/mL, AUC0–inf was 681 vs. 668 ng h/mL, plasma clearance was 4.40 vs. 4.49 (L/h/m2), respectively; harmonic means of t1/2 was 2.11 vs. 2.25 h.
Conclusions
This study showed that aprepitant in combination with palonosetron and dexamethasone was safe and effectively controlled CINV in sarcoma patients receiving ifosfamide and doxorubicin chemotherapy. Aprepitant may have a low potential to affect the pharmacokinetics of chemotherapeutic agents metabolized by CYP3A4.

Similar content being viewed by others
References
Hwang JS, Mehta AD, Yoon RS, Beebe KS. From amputation to limb salvage reconstruction: evolution and role of the endoprosthesis in musculoskeletal oncology. J Orthop Traumatol. 2014;15:81–6.
Patel SR, Vadhan-Raj S, Burgess MA, et al. Results of two consecutive trials of dose-intensive chemotherapy with doxorubicin and ifosfamide in patients with sarcomas. Am J Clin Oncol. 1998;21:317–21.
Judson I, Verweij J, Gelderblom H, et al. Doxorubicin alone versus intensified doxorubicin plus ifosfamide for first-line treatment of advanced or metastatic soft-tissue sarcoma: a randomized controlled phase 3 trial. Lancet Oncol. 2014;15:415–23.
Wagner MJ, Livingston JA, Patel SR, Benjamin RS. Chemotherapy for bone sarcoma in adults. J Oncol Pract. 2016;12:208–16.
Serrone L, Zeuli M, Gamucci T, Nardi M, Cognetti F. A phase II study of dose-intense ifosfamide plus epirubicin with hematopoietic growth factors for the treatment of patients with advanced soft tissue sarcomas; a novel sequential schedule. Cancer Chemother Pharmacol. 2001;47:206–10.
Tattersall FD, Rycroft W, Francis B, et al. Tachykinin NK1 receptor antagonists act centrally to inhibit emesis induced by the chemotherapeutic agent cisplatin in ferrets. Neuropharmacology. 1996;35:1121–9.
Hesketh PJ, Grunberg SM, Gralla RJ, et al. The oral neurokinin-1 antagonist aprepitant for the prevention of chemotherapy-induced nausea and vomiting: a multinational, randomized, double-blind, placebo-controlled trial in patients receiving high-dose cisplatin—the Aprepitant Protocol 052 Study Group. J Clin Oncol. 2003;21:4112–9.
Poli-Bigelli S, Rodrigues-Pereira J, Carides AD, et al. Addition of the neurokinin 1 receptor antagonist aprepitant to standard antiemetic therapy improves control of chemotherapy-induced nausea and vomiting. Results from a randomized, double-blind, placebo-controlled trial in Latin America. Cancer. 2003;97:3090–8.
Schmoll HJ, Aapro MS, Poli-Bigelli S, et al. Comparison of an aprepitant regimen with a multiple-day ondansetron regimen, both with dexamethasone, for antiemetic efficacy in high-dose cisplatin treatment. Ann Oncol. 2006;17:1000–6.
Warr DG, Hesketh PJ, Gralla RJ, et al. Efficacy and tolerability of aprepitant for the prevention of chemotherapy-induced nausea and vomiting in patients with breast cancer after moderately emetogenic chemotherapy. J Clin Oncol. 2005;23:2822–30.
Sanchez RI, Wang RW, Newton DJ, et al. Cytochrome P450 3A4 is the major enzyme involved in the metabolism of the substance P receptor antagonist aprepitant. Drug Metab Dispos. 2004;32:1287–92.
Fujita K. Cytochrome P450 and anticancer drugs. Curr Drug Metab. 2006;7:23–37.
Majumdar AK, McCrea JB, Panebianco DL, et al. Effects of aprepitant on cytochrome P450 3A4 activity using midazolam as a probe. Clin Pharmacol Ther. 2003;74:150–6.
Shadle CR, Lee Y, Majumdar AK, et al. Evaluation of potential inductive effects of aprepitant on cytochrome P450 3A4 and 2C9 activity. J Clin Pharmacol. 2004;44:215–23.
Durand JP, Gourmel B, Mir O, Goldwasser F. Antiemetic neurokinin-1 antagonist aprepitant and ifosfamide-induced encephalopathy. Ann Oncol. 2007;18:808–9.
Jarkowski A 3rd. Possible contribution of aprepitant to ifosfamide-induced neurotoxicity. Am J Health Syst Pharm. 2008;65:2229–31.
McCrea JB, Majumdar AK, Goldberg MR, et al. Effects of the neurokinin1 receptor antagonist aprepitant on the pharmacokinetics of dexamethasone and methylprednisolone. Clin Pharmacol Ther. 2003;74:17–24.
Zolezzi C, Ferrari S, Bacci G, Fasano MC, Sormani G, Pizzoferrato A. Determination of ifosfamide by HPLC using on-line sample preparation. J Chemother. 1999;11:69–73.
Campos D, Pereira JR, Reinhardt R, et al. Prevention of cisplatin induced emesis by the oral neurokinin-1 antagonist, aprepitant, in combination with granisetron and dexamethasone or with dexamethasone alone. J Clin Oncol. 2001;19:1759–67.
Navari R, Reinhardt R, Gralla RJ, et al. Reduction of cisplatininduced emesis by a selective neurokinin-1 receptor antagonist. N Engl J Med. 1999;340:190–5.
Cocquyt V, Van Belle S, Reinhardt R, et al. Comparison of L-758,298, a prodrug for the selective neurokinin-1 antagonist, L-754,030, with ondansetron for the prevention of cisplatin-induced emesis. Eur J Cancer. 2001;37:835–42.
Van Belle S, Lichinitser MR, Navari R, et al. Prevention of cisplatin induced acute and delayed emesis by the selective neurokinin-1 antagonists, L-758,298 and MK-869: a randomized controlled trial. Cancer. 2002;94:3032–41.
Loos WJ, de Wit R, Freedman SJ, et al. Aprepitant when added to a standard antiemetic regimen consisting of ondansetron and dexamethasone does not affect vinorelbine pharmacokinetics in cancer patients. Cancer Chemother Pharmacol. 2007;59:407–12.
Nygren P, Hande K, Petty KJ, et al. Lack of effect of aprepitant on the pharmacokinetics of docetaxel in cancer patients. Cancer Chemother Pharmacol. 2005;55:609–16.
Beulz-Riché D, Grudé P, Puozzo C, et al. Characterization of human cytochrome P450 isoenzymes involved in the metabolism of vinorelbine. Fundam Clin Pharmacol. 2005;19:545–53.
Marre F, Sanderink GJ, de Sousa G, Gaillard C, Martinet M, Rahmani R. Hepatic biotransformation of docetaxel (Taxotere) in vitro: involvement of the CYP3A subfamily in humans. Cancer Res. 1996;56:1296–302.
Acknowledgements
The authors would like to thank the patients who participated in this study.
Funding
Sponsorship for this study and article processing charges were funded by the Clinical Research Physician Program of Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei province, China. All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis.
Medical Writing Assistance
Medical writing assistance was provided by Fang Xiefan, PhD, from Charles River Laboratories, INC. 6995 Longley Lane, Reno, NV 89511, USA. No funding was received for this assistance.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosures
Xiong Jie, Zhao Guifang, Yang Shengli, and Chen Jing declare that they have no conflict of interest.
Compliance with Ethics Guidelines
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Data Availability
The data sets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced Digital Features
To view enhanced digital features for this article go to https://doi.org/10.6084/m9.figshare.7479701.
Rights and permissions
About this article
Cite this article
Xiong, J., Zhao, G., Yang, S. et al. Efficacy, Tolerability and Pharmacokinetic Impact of Aprepitant in Sarcoma Patients Receiving Ifosfamide and Doxorubicin Chemotherapy: A Randomized Controlled Trial. Adv Ther 36, 355–364 (2019). https://doi.org/10.1007/s12325-018-0862-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-018-0862-2