Abstract
Introduction
This study evaluated patients’ experiences with fluticasone furoate/vilanterol (FF/VI) combination therapy in UK patients with asthma or chronic obstructive pulmonary disease (COPD).
Methods
Participants aged ≥ 18 years, with self-reported, physician-diagnosed asthma or COPD (≥ 1 year) who had been receiving FF/VI (≥ 3 months) were recruited from UK primary care. This two-phase, mixed-methods study consisted of a semi-structured, telephone-interview phase (qualitative) and a self-completed online/paper-survey phase (quantitative).
Results
The telephone-interview phase included 50 individuals [asthma, n = 25; COPD, n = 25; mean age (SD) 56.7 years (13.3); 50% female]. Of these, 21 with asthma reported that their condition was stable/well controlled and 13 with COPD felt their condition was manageable. Most participants found FF/VI easy to use (asthma, 25; COPD, 23), easy to integrate into their daily routine (asthma, 25; COPD, 24), and able to control symptoms for ≥ 24 h (asthma, 14; COPD, 16). During the survey phase, 199 individuals were recruited [asthma, n = 100; COPD, n = 99; mean age (SD) 63.6 years (15.1); 59.3% female]. Most participants were satisfied/very satisfied with the efficacy of FF/VI in terms of all-day symptom relief (asthma, 84%; COPD, 75%) and found FF/VI easy/very easy to fit into their daily routine (asthma, 99%; COPD, 96%), easy/very easy to use (asthma, 97%; COPD, 92%), and convenient/very convenient to take as instructed (asthma, 95%; COPD, 93%). Significantly more individuals with asthma (87% versus 46%, P < 0.001) and numerically more individuals with COPD (84% versus 76%, P = 0.055) were satisfied/very satisfied with FF/VI compared with their most recent previous maintenance medication.
Conclusion
The majority of individuals in this study had confidence in FF/VI and were satisfied or very satisfied with various key attributes of the treatment.
Similar content being viewed by others
Introduction
Asthma and chronic obstructive pulmonary disease (COPD) are common respiratory conditions in the UK [1, 2]. Current treatment guidelines for adults with asthma recommend inhaled corticosteroids (ICS) as regular preventer therapy, with “step-up” therapy [such as an inhaled long-acting β2-agonist (LABA)] as necessary [3]. ICS/LABA treatments are also recommended as an option for patients with COPD [4]. ICS/LABA treatments are typically administered twice daily (e.g., fluticasone propionate/salmeterol) [5].
Both asthma and COPD can significantly affect the lives of people living with these conditions, who may experience substantial limitations in quality of life (QoL) and significant negative emotional impacts [6,7,8,9]. Shortness of breath is a key symptom of both asthma and COPD, which individuals typically report as the worst aspect of their condition [10]. Notable physical limitations associated with asthma and COPD include disturbed sleep and a reduced ability to socialize and undertake desired physical activities, while emotional impacts range from embarrassment to depression and fear [10,11,12]. Key attributes for medication in the treatment of asthma and COPD are the ability to reduce symptom impact and to enable undisturbed sleep [10, 13].
Fluticasone furoate/vilanterol (FF/VI) 100/25 µg is the first once-daily, inhaled ICS/LABA combination for the maintenance treatment of asthma [14, 15] and COPD [16, 17]. FF/VI is delivered via the ELLIPTA inhaler, a device that has been shown to be preferred and considered easy to use by patients in clinical studies [18,19,20]. The once-daily administration and 24-h efficacy [21, 22] of FF/VI may be associated with clinical benefits to patients in addition to convenience, such as reduced nocturnal symptoms and related improvements in sleep and QoL. However, patient perceptions of FF/VI and the potential impact of treatment on daily life are not fully understood.
This two-phase study aimed to evaluate participants’ experiences with, and perceptions of, FF/VI in the treatment of asthma and COPD.
Methods
Study Design and Objective
In the first phase, we assessed the experiences of individuals who were receiving treatment with FF/VI via semi-structured interviews. In the second phase, we used a patient satisfaction survey to quantify participants’ experiences and preferences with FF/VI on specific aspects of their treatment, including a comparison with their most recent previous inhaler treatment.
The study protocol and all study materials were reviewed and approved by the NHS Health Research Authority, UK (research ethics committee reference 16/LO/0836l; IRAS project ID 206320).
Participants
Participants with asthma or COPD were recruited through UK primary care on the basis of referrals from healthcare professionals (HCPs). Key inclusion criteria were age ≥ 18 years; a diagnosis of asthma, COPD, or asthma–COPD overlap (ACO) from a physician for ≥ 1 year; and current prescription of FF/VI for asthma or COPD and use of FF/VI for ≥ 3 months. Individuals could only participate in one phase of the study.
A broad participant sample for each condition was sought in both phases and based on age, gender, and disease control; asthma control and COPD impact were assessed by the Asthma Control Test™ (ACT) [23] and the COPD Assessment Test™ (CAT) [24], respectively. In total, 50 participants (asthma, n = 25; COPD, n = 25) were recruited for phase 1 (interviews) and 200 participants (asthma, n = 100; COPD, n = 100) were recruited for phase 2 (survey). Written, verbal, or electronic (via tick boxes) informed consent was obtained from all participants.
Phase 1: Qualitative Interviews
Procedure
Prior to the interview, participants were asked socio-demographic questions and completed the ACT [23] or CAT [24]. Any participant with ACO was interviewed about COPD and completed the CAT. The ACT scoring range is 5–25; scores < 20 indicate asthma that has not been controlled during the preceding 4 weeks [25]. The CAT scoring range is 0–40, with scores > 20 representing high disease impact and scores < 10 representing low disease impact [26, 27].
Participants completed individual, 60-min telephone interviews, conducted according to the approved individual interview guides. Question topics included frequency of use of FF/VI, thoughts on FF/VI as a once-daily medication, ease of use of FF/VI, current condition and symptoms, symptom control, length of control, change in QoL, impact of FF/VI on sleep, side effects, and confidence in FF/VI. Some patients were not asked every question.
Safety
This study was non-interventional and therefore presented minimal risk to participants. Any adverse events suspected to be associated with FF/VI were reported to the GSK Central Safety Department.
Analysis
Socio-demographic and ACT/CAT data were summarized using descriptive statistics. Interviews were transcribed verbatim and analyzed using a qualitative software tool, MAXQDA (v11.0.4 or above, VERBI GmbH 2015). A codebook based on the interview guide was developed and used to code transcripts; codes were analyzed using thematic analysis [28]. The most common themes that arose from the qualitative interviews were translated into closed-ended survey questions.
Phase 2: Quantitative Patient Surveys
The survey comprised quantitative questions on use of FF/VI (including frequency, administration timings, and ease/convenience) and satisfaction with the impacts of FF/VI [including efficacy (speed of onset, duration of symptom relief) and impacts on work, sleep, and QoL]. Satisfaction was rated on a 5-point scale (ranging from very dissatisfied to very satisfied). Participants also stated their most recent previous asthma/COPD maintenance medication prior to FF/VI and, unless FF/VI was their first medication, answered the same set of questions as for FF/VI. The order of the FF/VI and recent medications sections were randomized to control for any order effects relating to the interview questions. Additionally, three open-ended questions were included at the end of the FF/VI section that asked if there was anything the participant would rate as more positive/negative about FF/VI, and whether they would like to continue taking FF/VI and why. The content of the survey was developed using the results of the phase 1 qualitative interviews conducted with asthma and COPD patients prescribed FF/VI. A pilot version of the survey was tested in cognitive interviews; further details are available in the online supplement.
Main Survey Procedure
In addition to questions about FF/VI and recent inhaler medication, participants were asked socio-demographic questions and completed the ACT or CAT [23, 24]. To ensure a broad sample, participants were grouped by disease control/impact according to ACT score [uncontrolled asthma (< 15); not well or partly controlled asthma [15–19]; controlled asthma (≥ 20)] or CAT score [high-impact COPD (> 20); medium-impact COPD [10–20]; low-impact COPD (< 10)], with patients recruited proportionately to each group whenever possible. Participants completed the survey online or at their primary care site via tablet. Paper versions were available for individuals who were unable to travel or access the online survey.
Analysis
Demographic data, ACT/CAT scores, and survey responses were summarized using descriptive statistics. Quantitative analysis was undertaken using SPSS v19.0 and v24.0, with independent group comparisons (asthma versus COPD, site-complete versus self-complete) made using chi-squared statistics with testing for linear trend. Wilcoxon matched pairs signed-rank tests were used to compare aspects of FF/VI and recent maintenance medication within asthma and COPD groups.
Responses to the three free-text items were analyzed using a qualitative software tool, MAXQDA (v11.0.4 or above, VERBI GmbH 2015). Each item was analyzed using thematic analysis.
Compliance with Ethics Guidelines
This was a non-interventional observational study that did not alter the use or dosage of any treatment or alter participant perspectives in any way, and was not required to be registered on a public database. The study is posted on the GSK Clinical Study Register https://www.gsk-clinicalstudyregister.com/ Study No. 204888. All patients provided informed consent immediately before beginning the survey using the paper or online method that they used for the survey. The cognitive interview stage of the survey was reviewed and approved by an institutional review board (Salus IRB; protocol number 0018-0681). The survey stage of the study was approved by the NHS Health Research Authority (research ethics committee reference 16/LO/0836l; IRAS project ID 206320).
Results
Phase 1: Qualitative Interviews
Participants
In total, 50 participants completed the qualitative interviews [asthma, n = 25; COPD, n = 25 (COPD population includes one participant with ACO)]. The mean [standard deviation (SD)] age of participants with asthma or COPD was 52.6 (14.4) and 63.1 (9.8) years, respectively. Participants’ mean (SD) disease durations were 20.84 (15.85) years for asthma and 10.42 (6.78) years for COPD; 24% of participants with asthma and 48% with COPD were current smokers (Table 1).
The mean (SD) CAT score for participants with COPD was 22.40 (9.50), indicating a high impact of disease. The mean (SD) ACT score for participants with asthma was 19.72 (4.61), with 15 patients (60%) having ACT scores > 19.
Participants had been prescribed FF/VI for mean (SD) of 0.99 (0.45) years. Ventolin/salbutamol was the next most commonly taken medication. Table S1 contains a full list of current and previous medications.
Participants’ Usage of FF/VI
All but one participant took FF/VI once daily (Table 2). One participant with asthma took FF/VI twice daily on the basis of a recommendation from their doctor, but reported that once-daily dosing would be preferable. Most considered FF/VI easy to use (asthma, n = 25; COPD, n = 23) and felt that FF/VI could be integrated into their daily routine without disruption (asthma, n = 24; COPD, n = 25). Many participants (asthma, n = 19; COPD, n = 15) indicated that they preferred taking a once-daily maintenance medication rather than a more frequent dosage.
Nine participants (asthma, n = 5; COPD, n = 4) considered that a physical aspect of FF/VI affected their frequency of use (Table 2), with the most frequently reported response being that the medication left a powdery taste in the mouth (asthma, n = 1; COPD, n = 3).
Three participants with asthma and five with COPD reported feeling the effects of FF/VI immediately; however, 12 participants with asthma and four participants with COPD could not feel the medication taking effect (Table 2).
Participant Perceptions of FF/VI: Symptoms and Disease Control
The majority of participants with asthma (n = 21) considered that their condition was currently well managed and controlled, whereas only 13 participants with COPD considered their current condition and symptoms to be manageable (Table 2).
A high proportion of participants (asthma, n = 18; COPD, n = 16) reported that their symptoms were controlled after taking one dose of FF/VI. Of those whose asthma symptoms were not fully controlled, all seven indicated that they felt an improvement in symptom control since beginning treatment with FF/VI. Of the seven patients with COPD that were asked, five felt their symptoms were controlled better after a dose of FF/VI compared with previous treatments (Table 2). When asked about their symptoms since starting on FF/VI, one participant with asthma stated, “I know that I feel better in myself, I know that I’m breathing easier and I know it’s only since I started taking [FF/VI] so I can only assume it’s the [FF/VI] that’s done this”. Another participant noted, “I do still have episodes of feeling chesty, what I class as chesty and I still cough a lot which is another symptom of asthma so there are things that still go on as part of the asthma, they never 100% go away”.
Eleven participants with asthma and 16 with COPD felt that their symptoms were controlled all day, while three participants with asthma felt their symptoms were possibly controlled for longer than 24 h (Table 2). Seventeen participants with asthma and 11 with COPD considered that the length of symptom control was “better” with FF/VI compared with previous medications, although five participants with COPD considered that symptom control with FF/VI was the same. Some COPD participants were not sure whether their symptoms were controlled after a dose of FF/VI (n = 2), felt their symptoms were sometimes controlled (n = 1), reported that FF/VI treatment “doesn’t really make a lot of difference” to symptom control (n = 1), and felt unable to compare FF/VI with their previous treatment because of the number of other medications they were taking (n = 2).
Medications mentioned in comparison to FF/VI via the ELLIPTA inhaler included Becotide, Seretide, Symbicort, Clenil, beclomethasone, Qvar, and salbutamol by asthma patients, and Seretide, Genuair, Spiriva, and Fostair by COPD patients.
Effects of FF/VI on Participants’ QoL
The effect of FF/VI on participants’ daily activities varied across the two conditions, ranging from unchanged (n = 2) to improved (n = 2; because of reduced reliance on reliever inhaler) in the asthma group and worsening (n = 2; because of COPD deterioration) to improved (n = 3) in the COPD group. The majority of participants questioned about physical activities reported improvements (asthma, n = 13; COPD, n = 15), whereas six asthma and seven COPD participants reported no change since starting treatment with FF/VI. Specific improvements included positive impact on walking, exercise, climbing the stairs, and the ability to take up new physical activities; generally, participants with asthma were able to do more and/or for longer, while participants with COPD found physical activities easier than before starting treatment with FF/VI. One participant with COPD stated, “if I take [FF/VI] in the morning […] I can do my normal activities for the day, that I wouldn’t be able to do normally anyway with my condition”. Nine participants with asthma and one participant with COPD felt that FF/VI had a positive impact on their social life; those with asthma felt more confident when socializing as they were either less concerned about having an asthma attack or less tired and breathless, and the participant with COPD reported that FF/VI made them feel safe and secure. Seven asthma and 10 COPD participants, respectively, reported no change in their social life since starting treatment with FF/VI.
More participants with asthma than COPD considered that their sleep quality had improved with FF/VI treatment (asthma, n = 13; COPD, n = 4), largely owing to a reduction in the number of nighttime awakenings due to symptoms. Nine participants with asthma and 12 with COPD reported no change in their sleep quality, two of whom reported still waking during the night. One participant with COPD considered that their sleep quality had worsened since commencing FF/VI treatment.
The majority of participants with asthma (n = 18) and COPD (n = 15) did not report any side effects associated with treatment with FF/VI. Of the side effects that were reported, the most frequent were dry mouth (asthma, n = 4; COPD, n = 7) and thrush (asthma, n = 3; COPD, n = 2), none of which were reported as serious adverse events. When questioned further, seven participants with asthma and five with COPD indicated that avoiding side effects was important to them.
The majority of participants in both groups (asthma, n = 24; COPD, n = 23) felt confident with FF/VI treatment and, furthermore, 17 participants with asthma reported feeling “very confident” in their treatment. The most frequent reasons for confidence in FF/VI were the control of symptoms (asthma, n = 12; COPD, n = 14), reduced usage of their reliever medication (asthma, n = 3), increase in personal confidence (asthma, n = 3), and ease of use/convenience (COPD, n = 3). Two participants with COPD reported not feeling confident in FF/VI treatment because they did not feel the treatment had made a difference to their condition (n = 1) or as a result of medication side effects (n = 1). In general, however, participants reported a positive experience with FF/VI, with 20 participants with asthma and 15 with COPD considering FF/VI to be favorable compared with previous treatments. Further results of the impact of FF/VI in phase 1 are included in Table 2 and the online supplement.
Phase 2: Quantitative Survey
Participants
Two hundred participants completed the patient survey [asthma, n = 100; COPD, n = 100 (COPD population includes four participants with ACO)], of whom 197 (98.5%) completed the survey either online (n = 152) or via a tablet at their clinical site (n = 45). Two participants (1.5%) completed a paper version of the survey. Data from one participant with COPD (0.5%) who completed the paper survey was excluded from analysis because they received assistance from a family member in completing the survey; all other participants read and responded to either the online or paper survey themselves. Forty-seven participants (23.6%) completed the survey with assistance from the research team [asthma, n = 15 (15.0%); COPD n = 32 (32.3%)]; these individuals were generally older than those who self-completed the survey (mean age 69.5 versus 61.7 years, respectively). In total, data from 199 participants were analyzed (asthma, n = 100; COPD, n = 99).
The demographics and clinical characteristics of participants are shown in Table 1. The mean (SD) age of participants with asthma or COPD was 57.2 (17.0) and 70.0 (9.2) years, respectively. Participants with asthma had a mean (SD) disease duration of 23.9 (19.4) years, and those with COPD had a duration of 8.1 (8.3) years; 14.0% of participants with asthma and 23.2% with COPD were current smokers. The mean (SD) ACT score for participants with asthma was 19.0 (4.6), and 56.0% had an ACT score ≥ 20 (controlled). The mean (SD) CAT score for participants with COPD was 17.7 (9.5), and 22.2% had a CAT score < 10 (low impact). At the time of the survey, rescue medication was being taken by a higher number of individuals with COPD (92.9%) compared with asthma (72.0%).
Participants’ Usage of FF/VI
Participants with asthma had been taking FF/VI for a mean (SD) of 1.4 (1.0) years and those with COPD for 1.9 (1.9) years (Table 3). The majority of participants in both groups reported taking FF/VI once daily (asthma, 86.0%; COPD, 82.8%) and as part of their morning routine (asthma, 87.0%; COPD, 93.9%). Almost all participants reported that FF/VI was easy or very easy to use (asthma, 97.0%; COPD, 91.7%) and fit into their daily routine (asthma, 99.0%; COPD, 96.0%), and the majority found it convenient or very convenient to take as instructed (asthma, 95.0%; COPD, 92.9%).
Participants’ Satisfaction with FF/VI
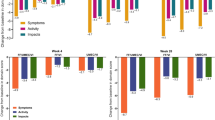
Most participants reported being satisfied or very satisfied with the frequency at which FF/VI was taken (asthma, 92.0%; COPD, 80.8%) and its ability to control their symptoms (asthma, 91.0%; COPD, 74.7%). The majority of participants were satisfied or very satisfied with the speed of onset (asthma, 89.0%; COPD, 74.7%) and duration of FF/VI’s effect (asthma, 90.0%; COPD, 75.8%), and were confident or very confident that it would relieve their symptoms all day (asthma, 84.0%; COPD, 70.7%). In almost all cases, the percentage of participants reporting satisfaction with the attributes of FF/VI was higher among participants with asthma than with COPD; some of these differences were statistically significant (Table 3).
Over three quarters of participants with asthma were satisfied or very satisfied with their ability to sleep through the night while taking FF/VI (78.0%) and with the level of impact FF/VI had on their ability to do physical or social activities (77.0% and 87.0%, respectively). For patients with COPD, 56.6% were satisfied or very satisfied with their ability to sleep through the night while taking FF/VI, with similar levels of satisfaction observed regarding the level of impact that FF/VI had on their ability to do physical or social activities (54.6% and 59.6%, respectively) (Table 3).
The majority of participants in both disease groups reported being satisfied or very satisfied with their overall experience of using FF/VI (asthma, 90.0%; COPD, 84.8%) (Table 3).
Most patents (asthma, 93.0%; COPD, 92.9%) reported that they would like to continue using FF/VI. Sample free-text responses to questions on participants’ experience of FF/VI are included in the supplementary materials.
Participants’ Satisfaction with FF/VI Compared with Their Most Recent Inhaled Medication
Fifty-four participants with asthma (54.0%) reported recent use of another maintenance inhaler medication for a mean (SD) duration of 5.8 (5.0) years. Sixty-three participants with COPD (63.6%) reported recent use of another maintenance inhaler medication for a mean (SD) duration of 4.2 (4.6) years. The most recent medications taken, most of which participants reported taking twice daily (asthma, 68.5%; COPD, 25.4%), are provided in Table 4.
Compared with their previous maintenance medication, significantly more participants with asthma were satisfied with the frequency of use of FF/VI (P = 0.001) and reported that FF/VI fitted more easily into their daily routine (P = 0.001), was more convenient to take as instructed (P = 0.009), and easier to use (P = 0.003), and that they forgot to take FF/VI less often (P = 0.003). Conversely, participants with COPD did not report significant advantages for FF/VI over their most recent medication; however, a numeric trend towards an advantage was observed (Table 5).
Participants with asthma were also significantly more satisfied with the efficacy of FF/VI compared with their recent maintenance medication, including time to onset of effect, symptom control, and post-dose duration of effect, and they felt confident that their symptoms would be relieved all day (all P < 0.001). Participants with asthma were also significantly more satisfied with the QoL impacts of FF/VI compared with their recent maintenance medication in terms of the impacts on their ability to perform physical and social activities, and their ability to sleep through the night (all P < 0.001) (Table 5).
Compared with their previous medication, participants with COPD were significantly more satisfied with the speed of onset of effect of FF/VI (P = 0.008), its impact on sleep quality (P = 0.031), the protection it conferred against triggers of exacerbations/flare-ups (P = 0.003), and how anxious/depressed they felt about their condition (P = 0.035). There was, however, no significant difference in level of satisfaction with the ability of both medications to control symptoms, duration of symptom control, or level of confidence that symptoms would be relieved (all P ≥ 0.055) (Table 5).
A significantly greater percentage of participants with asthma were satisfied or very satisfied with their overall experience of FF/VI, compared with their recent previous maintenance inhaler (87.0% versus 46.3%, respectively; P < 0.001) (Table 5). For those with COPD, there was no significant difference in participant satisfaction between FF/VI and participants’ previous medication in terms of QoL impacts, including ability to sleep through the night (P ≥ 0.151) and impact on ability to do physical and social activities (P = 0.143 and P ≥ 0.151, respectively). Consequently, there was no significant difference in the number of COPD participants who reported being satisfied or very satisfied with their overall experience of FF/VI compared with their recent previous maintenance inhaler (84.1% versus 76.2%; P = 0.055).
Discussion
This two-phase study evaluated patients’ experiences with, and perceptions of, FF/VI in the treatment of asthma and COPD. In the qualitative interviews, nearly all patients had confidence in FF/VI as a treatment for their condition, primarily due to its efficacy. Most patients were satisfied with the once-daily dosage frequency of FF/VI and found the treatment easy to use. In general, participants reported a positive experience with FF/VI when compared with their previous treatments, most of which were twice-daily medications. In the quantitative survey, there was a high level of satisfaction with FF/VI among participants from both disease groups. Most participants were satisfied with the efficacy of FF/VI, with patients with asthma reporting greater satisfaction with the QoL impacts of FF/VI versus those with COPD. Individuals with asthma reported significantly more satisfaction with all aspects of FF/VI’s efficacy and QoL impacts compared with their recent previous maintenance medication, whereas participants with COPD only reported significantly more satisfaction with FF/VI’s speed of onset, protection against exacerbations, and impacts on sleep quality, and any anxiety/depression they felt about their condition.
Symptom control is one of the key considerations in the treatment of asthma and COPD [4]. In phase 1, most participants reported that FF/VI controlled their symptoms for at least 24 h. In phase 2, the majority of participants were satisfied with both the level and duration of disease control afforded by FF/VI, and most were confident that FF/VI would relieve their symptoms all day. These findings are consistent with previous studies, which have shown improvements in symptom control and health status with FF/VI compared with other treatments for asthma and COPD, with evidence of a 72-h duration of bronchodilator effect [14, 15, 29,30,31].
Compared with participants with COPD, participants with asthma were significantly more satisfied with the ability of FF/VI to control their symptoms; they were also significantly more confident that their symptoms would be relieved all day versus their previous inhaler, which was not the case for participants with COPD. The difference in perceptions between participants with asthma and those with COPD may be related to the chronic and persistent nature of COPD symptoms, with asthma symptoms potentially being more variable [3, 4].
Differences in the baseline characteristics of participants with COPD and those with asthma may also have influenced whether they considered their symptoms to be well managed. For example, participants with COPD were older (phase 1, 63.1 years; phase 2, 70.0 years) than participants with asthma (phase 1, 52.6 years; phase 2, 57.2 years) and took a higher mean number of medications (phase 1, 3.28 versus 2.12 medications). Additionally, more participants with controlled asthma versus uncontrolled asthma were recruited in phase 2, whereas fewer participants with low-impact COPD were included compared with any other COPD severity category. This imbalance in condition severity between the two disease arms resulted from a relative scarcity of candidates with uncontrolled asthma or low-impact COPD during recruitment. The lack of potential candidates in these disease categories suggests that the current sample may be representative of patients being prescribed FF/VI in primary care.
It should be noted that although most participants considered that their symptoms were controlled with FF/VI, relatively few participants in phase 1 (seven with asthma and five with COPD) reported an improvement in their symptoms since commencing this treatment. Almost all participants across both study phases reported that FF/VI was easy to use and easy to integrate into their daily routine, consistent with previous research that demonstrated that participants found the ELLIPTA inhaler easy and convenient to use [18,19,20]. Previous studies have shown that more patients with asthma and COPD preferred ELLIPTA over comparator inhalers [32,33,34].
In addition to the convenience of a once-daily regimen, the 24-h continuous efficacy profile of FF/VI has been shown to improve nocturnal asthma symptoms [35], which may consequently improve sleep quality and provide benefits in terms of greater daytime productivity and ability to conduct various activities. Compared with their most recent inhaler, participants in both disease groups were significantly more satisfied with FF/VI in terms of its impact on sleep quality. However, only participants with asthma were significantly more satisfied with the effect of FF/VI on their ability to sleep through the night versus their previous medication. These results are in agreement with previous studies, which have shown that asthma is associated with a negative impact on sleep quality [36], while for COPD, having 24-h symptoms is associated with worse dyspnea, health status, and sleep quality, and higher levels of anxiety and depression [37].
Medication side effects were included in the phase 1 interview questions. Over 70% of participants with asthma and over 60% of those with COPD did not report experiencing side effects with FF/VI. Moreover, any side effects that were reported were common for the treatment class.
Strengths of phase 1 of the study included the use of an interview guide, and questions that were designed to be non-leading, reducing the potential for interviewer bias, as well as the reasonably large sample size for a qualitative study (N = 50). Moreover, by its nature, the study aimed to uncover and increase our understanding of individuals’ experiences of their condition and associated treatments. This is important since these experiences are likely to impact on the effectiveness of the treatment and also reveal potential unmet individual needs and preferences.
There are some limitations to the current study, including the lack of randomization, preventing comparisons between FF/VI and previous treatments, and the fact that only participants who were currently taking FF/VI were recruited (minimum time on FF/VI was 3 months). This may have resulted in a participant sample that was weighted toward those with positive opinions about the treatment, given that individuals who had previously ceased treatment with FF/VI might have switched to a different medication because they were not satisfied with FF/VI. Furthermore, as participants were already taking FF/VI at study recruitment, it is not possible to determine whether their ACT or CAT scores had improved as a result of FF/VI treatment. Additionally, while current prescription of FF/VI was based on medical records, previous maintenance medications were self-reported and thus subject to recall bias. Future research including participants who have been switched away from FF/VI may therefore provide additional context to the patient preferences reported here. The focus on FF/VI during the interviews and survey may have also impacted the answers given by the participants, potentially encouraging participants to provide a positive portrayal of FF/VI. However, participants did not always report positive opinions about all aspects of FF/VI, and some participants, particularly those with COPD, presented negative views, perhaps as a consequence of the progressive nature of their disease.
Despite the number of participants in the study, ethnic diversity was limited; caution is therefore warranted when generalizing these findings to the wider population, particularly since the average age for participants with asthma was higher than average for adults with asthma in the UK [38]. Moreover, in phase 2, almost one quarter of the sample (23.6%) had difficulties understanding the survey, and therefore completed it with assistance from the research team. Statistically significant differences were evident between individuals with COPD who had assistance versus those who completed the survey alone; participants who required assistance were on average almost 21 years older and had lower satisfaction with FF/VI compared with those who self-completed. Although this suggests heterogeneity between the assisted and self-completing participants, as both groups still represent views from individuals using FF/VI, it would have been misleading to exclude these participants from the analysis simply because they found the survey difficult to navigate and complete. Future studies should aim to ensure that the characteristics of participants involved in pilot or test phases of a study are reflective of those who will take part in the main study, particularly given that statistical measures such as chi-squared do not account for the influence of factors such as age or gender.
In conclusion, this study showed that participants were generally satisfied with the level of symptom control provided by FF/VI and felt confident with this treatment. Many participants experienced 24-h symptom control, with limited side effects, and frequently reported improvements in physical activities with FF/VI.
Change history
03 September 2021
A Correction to this paper has been published: https://doi.org/10.1007/s12325-021-01901-9
References
Asthma UK. Asthma facts and statistics. 2017. https://www.asthma.org.uk/about/media/facts-and-statistics/. Accessed 2 Aug 2017.
Insights: COPD factsheet. 2015. http://intelesant.com/copd-factsheet-june-2015/. Accessed 2 Aug 2017.
Global Initiative for Asthma. Global strategy for asthma management and prevention. 2017. http://www.ginasthma.org. Accessed 2 Aug 2017.
Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management and prevention of COPD. 2017. http://goldcopd.org. Accessed 2 Aug 2017.
Masoli M, Weatherall M, Holt S, Beasley R. Moderate dose inhaled corticosteroids plus salmeterol versus higher doses of inhaled corticosteroids in symptomatic asthma. Thorax. 2005;60(9):730–4.
Carranza Rosenzweig JR, Edwards L, Lincourt W, Dorinsky P, ZuWallack RL. The relationship between health-related quality of life, lung function and daily symptoms in patients with persistent asthma. Respir Med. 2004;98:1157–65.
Disler RT, Green A, Luckett T, et al. Experience of advanced chronic obstructive pulmonary disease: metasynthesis of qualitative research. J Pain Symptom Manag. 2014;48:1182–99.
Harrison SL, Apps L, Singh SJ, Steiner MC, Morgan MD, Robertson N. ‘Consumed by breathing’—a critical interpretive meta-synthesis of the qualitative literature. Chronic Illn. 2014;10:31–49.
Hughes M, Dunne M. The living with asthma study: issues affecting the perceived health and well-being of Irish adults with asthma. Ir J Med Sci. 2016;85:115–20.
Svedsater H, Roberts J, Patel C, Macey J, Hilton E, Bradshaw L. Life impact and treatment preferences of individuals with asthma and chronic obstructive pulmonary disease: results from qualitative interviews and focus groups. Adv Ther. 2017;34:1466–81.
Globe G, Martin M, Schatz M, et al. Symptoms and markers of symptom severity in asthma-content validity of the asthma symptom diary. Health Qual Life Outcomes. 2015;13:21.
Kulich K, Keininger DL, Tiplady B, Banerji D. Symptoms and impact of COPD assessed by an electronic diary in patients with moderate-to-severe COPD: psychometric results from the SHINE study. Int J Chronic Obstr Pulm Dis. 2015;10:79–94.
Vukoja M, Kopitovic I, Milicic D, Maksimovic O, Pavlovic-Popovic Z, Ilic M. Sleep quality and daytime sleepiness in patients with COPD and asthma. Clin Respir J. 2016. https://doi.org/10.1111/crj.12528.
Bernstein DI, Bateman ED, Woodcock A, et al. Fluticasone furoate (FF)/vilanterol (100/25 mcg or 200/25 mcg) or FF (100 mcg) in persistent asthma. J Asthma. 2015;52(10):1073–83.
Bleecker ER, Lötvall J, O’Byrne PM, et al. Fluticasone furoate–vilanterol 100-25 mcg compared with fluticasone furoate 100 mcg in asthma: a randomized trial. J Allergy Clin Immunol Pract. 2014;2(5):553–61.
Boscia JA, Pudi KK, Zvarich MT, Sanford L, Siederer SK, Crim C. Effect of once-daily fluticasone furoate/vilanterol on 24-h pulmonary function in patients with chronic obstructive pulmonary disease: a randomized, three-way, incomplete block, crossover study. Clin Ther. 2012;34:1655–66.
Dransfield MT, Bourbeau J, Jones PW, et al. Once-daily inhaled fluticasone furoate and vilanterol versus vilanterol only for prevention of exacerbations of COPD: two replicate double-blind, parallel-group, randomised controlled trials. Lancet Respir Med. 2013;1:210–23.
Svedsater H, Dale P, Garrill K, Walker R, Woepse MW. Qualitative assessment of attributes and ease of use of the ELLIPTA™ dry powder inhaler for delivery of maintenance therapy for asthma and COPD. BMC Pulm Med. 2013;13:72.
Bollmeier SG, Prosser TR. Patient perspectives on fluticasone–vilanterol versus other corticosteroid combination products for the treatment of asthma. Patient Preference Adherence. 2016;10:825–36.
Grant AC, Walker R, Hamilton M, Garrill K. The ELLIPTA® dry powder inhaler: design, functionality, in vitro dosing performance and critical task compliance by patients and caregivers. J Aerosol Med Pulm Drug Deliv. 2015;28:474–85.
Woodcock A, Bleecker ER, Busse WW, et al. Fluticasone furoate: once-daily evening treatment versus twice-daily treatment in moderate asthma. Respir Res. 2011;12:160.
Hanania NA, Feldman G, Zachgo W, et al. The efficacy and safety of the novel long-acting β2 agonist vilanterol in patients with COPD: a randomized placebo-controlled trial. Chest. 2012;142:119–27.
Bayliss MS, Kosinski M, Turner-Bowker DM, Fortin E. Asthma Control Test™: a user’s guide. Lincoln: QualityMetric; 2003.
COPD Assessment Test: healthcare professional user guide. 2012. http://www.catestonline.org/images/UserGuides/CATHCPUser%20guideEn.pdf. Accessed 2 Aug 2017.
Nathan RA, Sorkness CA, Kosinski M, et al. Development of the Asthma Control Test: a survey for assessing asthma control. J Allergy Clin Immunol. 2004;113:59–65.
Jones PW, Harding G, Berry P, et al. Development and first validation of the COPD Assessment Test. Eur Respir J. 2009;34(3):648–54.
Jones PW, Tabberer M, Chen WH. Creating scenarios of the impact of COPD and their relationship to COPD Assessment Test (CAT) scores. BMC Pulm Med. 2011;11:42.
Braun V, Clark V. Using thematic analysis in psychology. Qual Res Psychol. 2006;3:77–101.
Vestbo J, Leather D, Diar Bakerly N, et al. Effectiveness of fluticasone furoate–vilanterol for COPD in clinical practice. N Engl J Med. 2016;375(13):1253–60.
Martinez FJ, Boscia J, Feldman G, et al. Fluticasone furoate/vilanterol (100/25; 200/25 μg) improves lung function in COPD: a randomised trial. Respir Med. 2013;107(4):550–9.
Braithwaite I, Williams M, Power S, et al. Randomised, double-blind, placebo-controlled, cross-over single dose study of the bronchodilator duration of action of combination fluticasone furoate/vilanterol inhaler in adult asthma. Respir Med. 2016;119:115–21.
van der Palen J, Thomas M, Chrystyn H, et al. A randomised open-label cross-over study of inhaler errors, preference and time to achieve correct inhaler use in patients with COPD or asthma: comparison of ELLIPTA with other inhaler devices. NPJ Prim Care Respir Med. 2016;26:16079.
von Schantz S, Katajavuori N, Antikainen O, Juppo A. Evaluation of dry powder inhalers with a focus on ease of use and user preference in inhaler-naïve individuals. Int J Pharm. 2016;509:50–8.
Yun Kirby S, Zhu CQ, Kerwin EM, Stanford RH, Georges G. A preference study of two placebo dry powder inhalers in adults with COPD: ELLIPTA® Dry Powder Inhaler (DPI) versus DISKUS® DPI. COPD. 2016;13:167–75.
Kerwin E, Barnes N, Gibbs M, et al. Fluticasone furoate/vilanterol once daily improves night-time awakenings in asthma patients with night symptoms; post hoc analyses of three randomized controlled trials. J Asthma. 2017. https://doi.org/10.1080/02770903.2017.1362429.
de Sanz Burgoa V, Rejas J, Ojeda P, Investigators of the Coste Asma study. Self-perceived sleep quality and quantity in adults with asthma: findings from the CosteAsma study. J Investig Allergol Clin Immunol. 2016;26:256–62.
Miravitlles M, Worth H, Soler Cataluña JJ, et al. Observational study to characterize 24-h COPD symptoms and their relationship with patient-reported outcomes: results from the ASSESS study. Respir Res. 2014;15:122.
British Lung Foundation. Asthma statistics. 2017. https://statistics.blf.org.uk/asthma. Accessed 18 Aug 2017.
Acknowledgements
The authors would like to thank all of the participants in this study, members of the Global Perspectives™ recruitment team, HCPs who referred patients and assisted in patient screening, and Katie Hall, who assisted with all stages of the study while at ICON Clinical Research UK, including protocol development, interviewing and analysis. Becotide, ELLIPTA, Flixotide, Incruse, Seretide, Serevent, and Ventolin are trademarks owned by, or licensed to, the GSK group of companies. Clenil and Fostair are trademarks owned by, or licensed to, Chiesi. Seebri and Onbrez are trademarks owned by, or licensed to, Novartis Pharmaceuticals. Genuair, Pulmicort and Symbicort are trademarks owned by, or licensed to, AstraZeneca. Qvar, DuoResp, and Spiromax are trademarks owned by, or licensed to, Teva Pharmaceuticals. Spiriva is a trademark owned by, or licensed to, Boehringer Ingelheim.
Funding
This study, the article processing charges and the Open access fee were funded by GSK (GSK study HO-15-15503/204888). All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis.
Medical Writing and/or Editorial Assistance
Editorial support in the form of development of the draft outline and manuscript drafts in consultation with the authors, editorial suggestions to improve draft versions of this paper, assembling tables and figures, collating author comments, copyediting, fact checking, referencing, and graphic services was provided by Jennifer Lawton, PhD, and David Mayes, MChem, of Gardiner-Caldwell Communications (Macclesfield, UK), and was funded by GSK.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, had full access to all of the data in this study, take responsibility for the integrity of the work as a whole, and have given final approval to the version to be published.
Disclosures
Henrik Svedsater is a GSK employee and holds GSK stocks. Helen A Doll is an employee of ICON Clinical Research UK, which has received funding from GSK. Magdalena Vanya is an employee of ICON plc, which has received funding from GSK. Jake Macey was an employee of ICON Clinical Research UK at the time of the study. Jake Macey is currently affiliated with Clinical Outcomes Assessment, DRG Abacus, Bicester, UK. Gail Miles and Lisa Bradshaw declare that they have no competing interests.
Compliance with Ethics Guidelines
This was a non-interventional observational study that did not alter the use or dosage of any treatment or alter participant perspectives in any way, and was not required to be registered on a public database. The study is posted on the GSK Clinical Study Register https://www.gsk-clinicalstudyregister.com/ Study No. 204888. All patients provided informed consent immediately before beginning the survey using the paper or online method that they used for the survey. The cognitive interview stage of the survey was reviewed and approved by an institutional review board (Salus IRB; protocol number 0018-0681). The survey stage of the study was approved by the NHS Health Research Authority (research ethics committee reference 16/LO/0836l; IRAS project ID 206320).
Data Availability
The GSK sponsored datasets generated during and/or analyzed during the current study can be requested by making an enquiry via http://www.clinicalstudydatarequest.com
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced digital content
To view enhanced digital content for this article go to https://doi.org/10.6084/m9.figshare.6854351.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Svedsater, H., Doll, H.A., Macey, J. et al. Evaluating the Impact and Benefits of Fluticasone Furoate/Vilanterol in Individuals with Asthma or COPD: A Mixed-Methods Analysis of Patient Experiences. Adv Ther 35, 1378–1399 (2018). https://doi.org/10.1007/s12325-018-0760-7
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-018-0760-7