Abstract
Introduction
FreeStyle Libre (Abbot Diabetes Care Ltd) has been launched as a novel glucose monitoring system called flash glucose monitoring (FGM) in Europe. Several reports are becoming available on its usefulness and safety. To date, however, reports from Asian countries have not been made available. In this study, we evaluated its usefulness in Japanese people with diabetes in terms of its mental well-being and patient satisfaction outcomes.
Methods
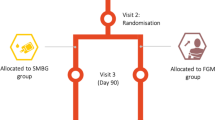
Individuals with type 1 and 2 diabetes treated with insulin were enrolled, and they performed self-monitoring of blood glucose. All participants were subjected to FGM for 14 days and compared for changes in mental well-being using the WHO-Five Well-Being Index (WHO-5) (1998 version) as well as in patient satisfaction using the Diabetes Treatment Satisfaction Questionnaire (DTSQ) before and after implementation of FGM.
Results
The study included a total of 80 subjects (type 1/2 diabetes, 57/23). The WHO-5 scores were significantly improved from 15.5 ± 4.1 at baseline to 17.2 ± 4.5 after implementation of FGM (P < 0.001); the DTSQ scores also were significantly improved from 24.8 ± 6.0 to 26.7 ± 5.2 (P = 0.001). In type 1 diabetes, both the WHO-5 and DTSQ scores were significantly improved from baseline (P = 0.001, P = 0.001), while neither the WHO-5 scores nor the DTSQ scores were improved in type 2 diabetes.
Conclusions
The study results suggest that FGM has the potential to improve mental well-being and treatment satisfaction among individuals with type 1 diabetes.
Similar content being viewed by others
References
Peyrot M, Rubin RR, Lauritzen T, et al. Psychosocial problems and barriers to improved diabetes management: results of the Cross-National Diabetes Attitudes, Wishes and Needs (DAWN) Study. Diabet Med. 2005;22:1379–85.
Amiel SA, Dixon T, Mann R, Jameson K. Hypoglycaemia in type 2 diabetes. Diabet Med. 2008;25:245–54.
Pouwer F, Hermanns N. Insulin therapy and quality of life. A review. Diabetes Metab Res Rev. 2009;25:S4–10.
Vijan S, Hayward RA, Ronis DL, Hofer TP. Brief report: the burden of diabetes therapy: implications for the design of effective patient-centered treatment regimens. J Gen Intern Med. 2005;20:479–82.
Bolinder J, Antuna R, Geelhoed-Duijvestijn P, Kröger J, Weitgasser R. Novel glucose-sensing technology and hypoglycaemia in type 1 diabetes: a multicentre, non-masked, randomised controlled trial. Lancet. 2016;388:2254–63.
Haak T, Hanaire H, Ajjan R, et al. Flash glucose-sensing technology as a replacement for blood glucose monitoring for the management of insulin-treated type 2 diabetes: a multicenter open-label randomized controlled trial. Diabetes Ther. 2017;8:55–73.
Aberer F, Hajnsek M, Rumpler M, et al. Evaluation of subcutaneous glucose monitoring systems under routine environmental conditions in patients with type 1 diabetes. Diabetes Obes Metab. 2017;19(7):1051–5. https://doi.org/10.1111/dom.12907.
McKnight JA, Gibb FW. Flash glucose monitoring is associated with improved glycaemic control but use is largely limited to more affluent people in a UK diabetes centre. Diabet Med. 2017;34:732.
Ólafsdóttir AF, Attvall S, Sandgren U, et al. A clinical trial of the accuracy and treatment experience of the flash glucose monitor freestyle libre in adults with type 1 diabetes. Diabetes Technol Ther. 2017;19:164–72.
Staehr Johansen K. The use of well-being measures in primary health care–the DepCare project; in World Health Organization, Regional Office for Europe: well-being measures in primary health care–the DepCare project. Geneva: World Health Organization; 1998.
Bradley C. The diabetes treatment satisfaction questionnaire: DTSQ. In: Bradley C, editor. Handbook of psychology and diabetes: a guide to psychological measurement in diabetes research and practice. Chur: Harwood Academic; 1994. p. 111–32.
Topp CW, Østergaard SD, Søndergaard S, Bech P. The WHO-5 Well-Being Index: a systematic review of the literature. Psychother Psychosom. 2015;84:167–76.
Inagaki H, Ito K, Sakuma N, et al. Reliability and validity of the simplified Japanese version of the WHO-Five Well-Being Index (S-WHO-5-J). Nihon Koshu Eisei Zasshi. 2013;60:294–301 (in Japanese).
de Wit M, Pouwer F, Gemke RJ, Delemarre-van de Waal HA, Snoek FJ. Validation of the WHO-5 Well-Being Index in adolescents with type 1 diabetes. Diabetes Care. 2007;30:2003–6.
Awata S, Bech P, Yoshida S, et al. Reliability and validity of the Japanese version of the World Health Organization-Five Well-Being Index in the context of detecting depression in diabetic patients. Psychiatry Clin Neurosci. 2007;61:112–9.
Hajos TR, Pouwer F, Skovlund SE, et al. Psychometric and screening properties of the WHO-5 Well-Being Index in adult outpatients with type 1 or type 2 diabetes mellitus. Diabet Med. 2013;30:e63–9.
Furuya M, Hayashino Y, Tsujii S, Ishii H, Fukuhara S. Comparative validity of the WHO-5 Well-Being Index and two-question instrument for screening depressive symptoms in patients with type 2 diabetes. Acta Diabetol. 2013;50:117–21.
Roy T, Lloyd CE. Epidemiology of depression and diabetes: a systematic review. J Affect Disord. 2012;142:S8–21.
Lloyd CE, Pambianco G, Orchard TJ. Does diabetes-related distress explain the presence of depressive symptoms and/or poor self-care in individuals with type 1 diabetes? Diabet Med. 2010;27:234–7.
Health psychology research web site. http://www.healthpsychologyresearch.com. Accessed 8 May 2017.
Bergenstal RM, Klonoff DC, Garg SK, et al. Threshold-based insulin-pump interruption for reduction of hypoglycemia. N Engl J Med. 2013;369:224–32.
Polonsky WH, Hessler D. What are the quality of life-related benefits and losses associated with real-time continuous glucose monitoring? A survey of current users. Diabetes Technol Ther. 2013;15:295–301.
Hirose M, Beverly EA, Weinger K. Quality of life and technology: impact on children and families with diabetes. Curr Diab Rep. 2012;12:711–20.
Beck RW, Riddlesworth T, Ruedy K, et al. Effect of continuous glucose monitoring on glycemic control in adults with type 1 diabetes using insulin injections: the DIAMOND randomized clinical trial. JAMA. 2017;317:371–8.
Papanas N, Tsapas A, Papatheodorou K, et al. Glycaemic control is correlated with well-being index (WHO-5) in subjects with type 2 diabetes. Exp Clin Endocrinol Diabetes. 2010;118:364–7.
Ishii H, Anderson JH Jr, Yamamura A, Takeuchi M, Ikeda I. Improvement of glycemic control and quality-of-life by insulin lispro therapy: assessing benefits by ITR-QOL questionnaires. Diabetes Res Clin Pract. 2008;81:169–78.
Ishii H. Development and psychometric validation of the diabetes therapy-related QOL (DTR-QOL) questionnaire. J Med Econ. 2000;15:556–63.
Acknowledgements
Funding
This work was partially supported by Japan Society for the Promotion of Science KAKENHI Grant Number JP15K20697. Authors funded the article processing charges for this paper. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval for the version to be published. The authors would like to extend their heartfelt thanks to all patients for their participation in the study.
Disclosures
Rimei Nishimura has participated in speaker’s bureau/advisory panels for Astellas, Boehringer Ingelheim, Eli Lilly, Kissei, Medtronic, Novo Nordisk, Sanofi, and Takeda. Shin-ichi Harashima has participated in speaker’s bureau/advisory panels for Novo Nordisk, Sanofi, Eli Lilly, and Tanabe Mitsubishi, and has received research support from AstraZeneca. Tomoyuki Kawamura has participated in speaker’s bureau/advisory panels for Eli Lilly and Sanofi. Keiko Koide has participated in speaker’s bureau/advisory panels for Arkray, Johnson & Johnson, and TERUMO. Kazunori Utsunomiya has received research support from Arkray, Astellas, Boehringer Ingelheim, Kissei, Novo Nordisk, Ono, Taishyo, Tanabe-Mitsubishi, and Terumo, and has participated in speaker’s bureau/advisory panels for Eli Lilly, MSD, Taishyo, and Sanofi. Nobuya Inagaki received research support from Tanabe Mitsubishi, MSD, Ono, Takeda, Dainippon Sumitomo, Daiichi Sankyo, Kyowa Hakko Kirin, Japan Tabacco, Boehringer Ingelheim, Novartis, Sanofi, Taisho Toyama, and Astellas. Yoshihito Atsumi has participated in speaker’s bureau/advisory panels for Arkray, Astellas, Eli Lilly, MSD, Novo Nordisk, Ono, Sanofi Taishyo, and Tanabe-Mitsubishi. Sumie Mitsuishi, Daisuke Tsujino, and Akiko Nishimura have nothing to disclose.
Compliance With Ethics Guidelines
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964, as revised in 2013. Informed consent was obtained from all patients for being included in the study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced content
To view enhanced content for this article go to http://www.medengine.com/Redeem/6AFCF0607CBD7797.
Rights and permissions
About this article
Cite this article
Mitsuishi, S., Nishimura, R., Harashima, Si. et al. The Effect of Novel Glucose Monitoring System (Flash Glucose Monitoring) on Mental Well-being and Treatment Satisfaction in Japanese People with Diabetes. Adv Ther 35, 72–80 (2018). https://doi.org/10.1007/s12325-017-0649-x
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-017-0649-x