Abstract
Introduction
Using the recommended doses obtained from our previous phase 1 trial of a modified Saltz chemotherapy regimen for metastatic colorectal cancer (weekly irinotecan and bolus 5-fluorouracil/l-leucovorin for 3 weeks every 28 days), we performed the present phase 2 trial to evaluate efficacy and toxicity.
Methods
A total of 29 patients with metastatic colorectal cancer were included. Our modified Saltz regimen was administered. The primary endpoint was overall response rate.
Results
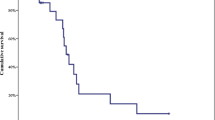
Of the 29 patients, 11 had previous chemotherapy. A partial response occurred in 11 patients, stable disease in 16 patients, and progressive disease in two patients. Disease control rate was 93.1%. Response rates with and without previous treatment were 18.2% and 50%, respectively. Median progression-free survival was 17.3 months. The main hematologic toxicities were leukopenia (22.6%) and neutropenia (45.2%). No treatment-related deaths occurred.
Conclusion
Our modified Saltz regimen exhibited sufficient efficacy, feasibility, and manageable toxicity as a therapeutic option for selected colorectal cancer patients.
Similar content being viewed by others
References
Matsuda T, Marugame T, Kamo K, Katanoda K, Ajiki W, Sobue T. Japan Cancer Surveillance Research Group. Cancer incidence and incidence rates in Japan in 2002: based on data from 11 population-based cancer registries. Jpn J Clin Oncol. 2008;38:641–648.
[No authors listed]. Modulation of fluorouracil by leucovorin in patients with advanced colorectal cancer: evidence in terms of response rate. Advanced Colorectal Cancer Meta-Analysis Project. J Clin Oncol. 1992;10:896–903.
[No authors listed]. Meta-analysis of randomized trials testing the biochemical modulation of fluorouracil by methotrexate in metastatic colorectal cancer. Advanced Colorectal Cancer Meta-Analysis Project. J Clin Oncol. 1994;12:960–969.
Grothey A, Sargent D, Goldberg RM, Schmoll HJ. Survival of patients with advanced colorectal cancer improves with the availability of fluorouracil-leucovorin, irinotecan, and oxaliplatin in the course of treatment. J Clin Oncol. 2004;22:1209–1214.
Saltz LB, Cox JV, Blanke C, et al. Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. Irinotecan Study Group. N Engl J Med. 2000;343:905–914.
Douillard JY, Cunningham D, Roth AD, et al. Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: a multicentre randomized trial. Lancet. 2000;355:1041–1047.
Fujishima H, Kikuchi I, Miyanaga O, et al. Phase I study of CPT-11 and bolus 5-FU/l-leucovorin in patients with metastatic colorectal cancer. Int J Clin Oncol. 2004;9:92–97.
Yoshino M, Ota K, Kurihara M, et al. Late phase II trial of high-dose l-leucovorin and 5-fluorouracil in advanced colorectal carcinoma. l-Leucovorin and 5-FU Study Group (Japan Eastern Group) [in Japanese]. Gan to Kagaku Ryoho. 1995;22:785–792.
Konishi K, Yabushita K, Taguchi T, et al. A late phase II trial of l-leucovorin and 5-fluorouracil in advanced colorectal cancer. l-Leucovorin and 5-FU Study Group (Japan Western Group) [in Japanese]. Gan to Kagaku Ryoho. 1995;22:925–932.
Shimada Y, Yoshino M, Wakui A, et al. Phase II study of CPT-11, a new camptothecin derivative, in metastatic colorectal cancer. CPT-11 Gastrointestinal Cancer Study Group. J Clin Oncol. 1993;11:909–913.
Goldberg RM, Sargent DJ, Morton RF, et al. A randomized controlled trial of fluorouracil plus leucovorin, irinotecan, and oxaliplatin combinations in patients with previously untreated metastatic colorectal cancer. J Clin Oncol. 2004;22:23–30.
Tournigand C, Andre T, Achille E, et al. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol. 2004;22:229–237.
Van Cutsem E, Köhne CH, Hitre E, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009;360:1408–1417.
Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2343.
Salts LB, Clarke S, Díaz-Rubio E, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer. J Clin Oncol. 2008;26:2013–2019.
Kabbinavar F, Schulz J, McCleod M, et al. Addition of bevacizumab to bolus fluorouracil and leucovorin in first-line metastatic colorectal cancer: results of a randomized phase II trial. J Clin Oncol. 2005;23:3697–3705.
Giantonio BJ, Catalano PJ, Meropol NJ, et al. Eastern Cooperative Oncology Group Study E3200. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol. 2007;25:1539–1544.
Vardy J, Engelhardt K, Cox K, et al. Long-term outcome of radiological-guided insertion of implanted central venous access port devices (CVAPD) for the delivery of chemotherapy in cancer patients: institutional experience and review of the literature. Br J Cancer. 2004;91:1045–1049.
Vescia S, Baumgärtner AK, Jacobs VR, et al. Management of venous port systems in oncology: a review of current evidence. Ann Oncol. 2008;19:9–15.
Inaba Y, Yamaura H, Sato Y, et al. Central venous access port-related complications in outpatient chemotherapy for colorectal cancer. Jpn J Clin Oncol. 2007;37:951–954.
Fuchs CS, Marshall J, Mitchell E, et al. Randomized, controlled trial of irinotecan plus infusional, bolus, or oral fluoropyrimidines in first-line treatment of metastatic colorectal cancer: results from the BICC-C Study. J Clin Oncol. 2007;25:4779–4786.
Author information
Authors and Affiliations
Corresponding author
Additional information
To view enhanced content go to www.advancesintherapy.com
Rights and permissions
About this article
Cite this article
Baba, E., Fujishima, H., Makiyama, A. et al. Phase 2 Study of Modified Irinotecan and Bolus 5-Fluorouracil/l-Leucovorin in Japanese Metastatic Colorectal Cancer Patients. Adv Therapy 29, 287–296 (2012). https://doi.org/10.1007/s12325-012-0002-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-012-0002-3