Abstract
Chronic myeloid leukemia (CML) is a clonal hematopoietic stem cell disorder characterized by the presence of a cytogenetic abnormality, the Philadelphia (Ph) chromosome. BCR-ABL tyrosine kinase inhibitors (TKIs) have revolutionized the treatment of chronic myeloid leukemia (CML). Patients who respond to treatment achieve a near-normal life expectancy. In this case report, we present the first case from Turkey of reversible follicular lymphoid hyperplasia (FLH) related to the use of a second-generation TKI, dasatinib. A peripheral blood specimen was evaluated by double-fusion fluorescence in situ hybridization (FISH) for cytogenetics and by quantitative RT–PCR (qPCR) for BCR-ABL1 mRNA transcripts. The cervical lymph node biopsy tissue was embedded in paraffin and sectioned following routine methods after fixation in neutral buffered formalin. Immunohistochemical staining was applied for further evaluation. A 64-year-old male CML patient with complete cytogenetic response (CCyR) and major molecular response (MMR) presented with lymphadenopathies accompanied by mild lymphocytosis following 3 months of dasatinib treatment. The patient was diagnosed with FLH. After termination of dasatinib, the lymphadenopathies regressed during follow-up. FLH should be considered a rare side effect of dasatinib. A watch and wait strategy following dasatinib treatment termination would be suitable.
Similar content being viewed by others
Introduction
Chronic myeloid leukemia (CML) is defined by the presence of a distinctive cytogenetic abnormality, the Philadelphia (Ph) chromosome, which is a translocation between chromosomes 9 and 22, t(9;22)(q34;q11.2), that leads to a BCR-ABL fusion gene. CML patients primarily present with overproduction of myeloid cells. Fatigue, splenomegaly, leukocytosis, thrombocytosis, and anemia are common clinical findings. Targeted inhibition of the oncogenic BCR-ABL tyrosine kinase has significantly changed CML therapy [1]. Dasatinib is a second-generation and more potent tyrosine kinase inhibitor (TKI) approved to treat first-generation TKI (imatinib)-resistant CML, and has a broader target landscape, including SRC, SYK, and TEC kinases. The most prevalent dasatinib-related adverse effect is pleural effusion, which has been reported in up to 20% of patients [2]. Nausea/vomiting, diarrhea, muscle cramps, and edema, as well as cardiac, pulmonary, metabolic/endocrine, and hepatic toxicities may also occur. Furthermore, lymphocytosis and immune-modulatory effects of dasatinib have been previously demonstrated in vivo and in vitro [3, 4]. Mustjoski et al. presented marked clonal lymphoproliferation caused by persistent expansion of cytotoxic T cells or natural killer-large granular lymphocyte (NK-LGL) cells in 22 patients during dasatinib therapy [5]. Here, we report a chronic-phase CML patient who developed a distinct form of follicular lymphoid hyperplasia (FLH) accompanied by peripheral lymphocytosis following dasatinib treatment by focusing on clinicopathological and radiographical findings.
Clinical history
A 64-year-old male patient was diagnosed with chronic-phase CML with a low-risk EUTOS long-term survival score (ELTS) following a work-up for fatigue, and imatinib was initiated [6]. There was a suboptimal response at 20 months of treatment with a BCR-ABL1 transcript level of 0.2%, and imatinib was replaced with dasatinib. The patient presented with progressive bilateral nontender cervical, axillary, and inguinal lymphadenopathies after 3 months of treatment. At the time of the lymph node enlargements, the patient was in complete cytogenetic response (CCyR) and major molecular response (MMR) with mild lymphocytosis. The physical examination demonstrated no signs of local or systemic infections. Serology for viral infections, autoimmune disorders, and toxoplasma revealed no active disease. The computed tomography (CT) component of fluorine-18-fluorodeoxyglucose (18F-FDG) positron emission tomography/CT (PET/CT) showed bilateral enlarged cervical lymph nodes (Fig. 1a) without pathological 18F-FDG uptake in the axial fusion image of 18F-FDG PET/CT (Fig. 1b). The coronal PET/CT fusion image did not demonstrate pathological uptake in any other parts of the body (Fig. 1c). The patient gave consent for right cervical lymph node biopsy and publication of findings.
a Axial computed tomography (CT) demonstrates bilateral-enlarged cervical lymph nodes (arrows). b Axial 18flor-fluorodeoxyglucose (18F-FDG) positron emission tomography (PET) and CT fusion images do not exhibit pathological uptake of these enlarged cervical lymph nodes (arrows). c Coronal PET/CT fusion image does not demonstrate pathological uptake in any other parts of the body
Materials and methods
Cytogenetic evaluation of the peripheral blood specimen was performed by double-fusion fluorescence in situ hybridization (FISH) with a cut-off level of 5% (HealthCare Biotech, China). Quantitative RT-PCR (qPCR) was performed to establish the presence of quantifiable BCR-ABL1 mRNA transcripts.
Histologic and immunophenotypic studies
After fixation in neutral buffered formalin, the lymph node was embedded in paraffin and sectioned following routine methods. The sections were stained with hematoxylin and eosin (H&E). Immunohistochemical staining with CD3 (clone: ZM45) (Zeta Corporation, CA, USA), CD5 (clone: CD5/54/F6) (ScyTek Laboratories Inc, UT, USA) bcl-2 (clone: 100/D5) (ScyTek Laboratories Inc, UT, USA), CD68 (clone: KP1) (Zeta Corporation, CA, USA), bcl-6 (clone: LN22) (Biocare Medical, CA, USA), CD20 (clone: L26) (Biocare Medical, CA, USA), CD10 (clone: 56C6) (Biocare Medical, CA, USA), Tdt (clone: ZM51) (Zeta Corporation, CA, USA) and Ki-67(clone: SP6) (Zeta Corporation, CA, USA) primary antibodies was applied on 2-µm thickness tissue sections by the manual indirect streptavidin–biotin peroxidase method.
De-paraffinization and rehydration stage
Slices were kept at 60 °C for one night in the incubator. They were prepared using xylene, 100% ethanol, and 96% ethanol for 15 min each. Afterwards, slices were washed using distilled water.
Antibody recovery stage
A citrate buffer at pH 6 was prepared using 20 ml citrate buffer and 180 ml of distilled water. The slices were placed 3 times for 5 min in a 700 W microwave. To return the slices to room temperature, they were kept in buffered citrate and EDTA (for Tdt) for 20 min. After washing them under distilled water, the tissue borders were framed using Pap-pen.
Antibody application
A 3% hydrogen peroxide (H2O2) was applied to prevent endogenic peroxidase for 15 min before tissue slices as put under distilled water. Isosmotic arrangement was made in phosphate-buffered saline (PBS) with a pH of 7.4. In order to prevent background staining, Ultra V block solution was applied for 5 min. After the solution was removed from the slices, primary antibodies were applied to each slice for 2 h. After PBS application, to stabilize he antibody-antigen complex, biotin was applied as a coenzyme for 30 min. PBS was reapplied, and a second coenzyme, streptavidin peroxidase, was applied for 30 min. The tissues were placed into PBS again. To visualize the connections, a chromogen (DAB) was applied for 5–9 min. Finally, the tissues were washed with distilled water, and Mayer’s hematoxylin solution was applied for 5 min for progressive staining.
Results
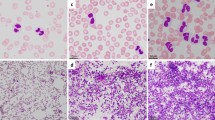
In low-magnification histopathological examination, the first remarkable finding was large lymphoid follicular structures with large and irregular germinal centers and expansion of the mantle zone. At high magnification, enlargement in the interfollicular area was accompanied by vascular proliferation. In immunohistochemical staining, strong membranous and nuclear staining supported reactivity with CD3, CD5, bcl-2, and CD68, which were positive for interfollicular and parafollicular areas; bcl-6, CD20, and Ki-67 were positive for follicular areas. There were invaginations of CD5 ( −) mantle zone cells to enlarged germinal centers in reactive secondary follicles (white arrow, Fig. 2a, b). Proliferative vessels in the perifollicular area were penetrating to the germinal centers (white arrow Fig. 2b). The germinal centers were disrupted clearly by vascular structures and mantle zone with CD20 staining (Fig. 2c). In a differential diagnosis of follicular lymphoma or follicular in situ lesions, the predominance of CD20 and Ki-67 in the germinal centers and mantle zones, the dominance of CD3 ( +) T-lymphocytes in the interfollicular areas, and bcl-2 and Tdt negativity whereas CD10 and bcl-6 positivity in the germinal centers were findings in favor of reactive hyperplasia (Fig. 2d, e, f, g). Following the diagnosis of FLH, dasatinib was discontinued and switched to nilotinib. The reactive lymphadenopathies regressed within 2 months. The patient is still in follow-up with CCyR and MMR.
a Invagination of mantle zone to germinal center (white arrow, hematoxylin and eosin (H&E) stain). b The penetration of proliferated vascular structures in perifollicular area to germinal center (white arrow, H&E stain). c The penetrations of mantle zone and vascular structures to germinal center (white arrows, CD20 stain). d CD10 positivity in germinal centers. e bcl-2 negativity in germinal centers. f bcl-6 positivity in germinal centers. g The similar distribution of Ki-67 in regular or irregular germinal centers
Discussion
The diagnostic work-up for any lymphadenopathy in a CML patient should include the evaluation of extramedullary blastic transformation of disease and the presence of a concurrent follicular lymphoma or follicular in situ lesion. In this case, the patient was in CCyR and MMR during the appearance of lymphadenopathies; furthermore, the 18F-FDG uptake of the lymph nodes was not pathologic, which excludes these causes. The histologic finding of drug-induced lymphadenopathy, especially with anticonvulsant therapy, was described previously [7]. Several published cases support the phenomenon of dasatinib-related lymphadenopathy [7,8,9,10], and to our knowledge, this is the first case from Turkey. In the histopathological examination, the predominant staining areas with CD20 were the germinal center, mantle zone, and partially parafollicular areas. Dominant staining with CD20 was not observed in the interfollicular areas. Although these findings were interpreted to favor reactive lymphoid hyperplasia, possible monoclonal B-cell-lymphocytosis and closely related in situ follicular neoplasia should not be ignored due to CD5 negativity in the same areas [11]. In our case, regression in lymphadenopathies after discontinuation of dasatinib confirmed the interpretation of reactivity. For a differential diagnosis, Castleman’s disease or progressive transformation of germinal centers (PTGC) might be considered [8]. We did not detect either penetrating vessels or hyalinization of vessel walls, which would suggest Castleman’s disease. In PTGC, germinal centers are markedly larger than normal, with indistinct margins and composed of follicular mantle lymphocytes and extensive follicular dendritic cells as well as tingible body macrophages. In our case, the germinal centers were enlarged, but they were irregular and had nearby vascular proliferations.
FLH is characterized by B lymphocyte stimulation. LYN, an SRC family protein, is expressed in B lymphocytes and is a regulator of B-cell antigen receptors via Atk/PKB signaling, contributing to B-cell activation [12, 13]. Here, the impact of dasatinib on SRC kinases and the Akt/PKB pathway could contribute to the initiation of FLH. Additionally, dasatinib-mediated lymphocytosis is partially related to the redistribution and accumulation of terminally differentiated T cells and memory B cells in the bloodstream [4].
We recommend considering physical examination and diagnostic work-up in patients treated with dasatinib to rule out FLH. Patients should be considered for dasatinib discontinuation and switched to another TKI if FLH is diagnosed.
References
Kantarjian H, Cortes J (2014) 101 - Chronic Myeloid Leukemia,Editor(s): John E. Niederhuber, James O. Armitage, James H. Doroshow, Michael B. Kastan, Joel E. Tepper, Abeloff's Clinical Oncology (Fifth Edition),Churchill Livingstone, 2014, Pages 1944–1957.e2,ISBN 9781455728657
Ottmann O, Dombret H, Martinelli G, Simonsson B, Guilhot F, Larson RA, Rege-Cambrin G, Radich J, Hochhaus A, Apanovitch AM, Gollerkeri A, Coutre S (2007) Dasatinib induces rapid hematologic and cytogenetic responses in adult patients with Philadelphia chromo- some positive acute lymphoblastic leukemia with resistance or intolerance to imatinib: interim results of a phase 2 study. Blood 110:2309–2315
Schiffer CA, Cortes JE, Hochhaus A, Saglio G, le Coutre P, Porkka K, Mustjoki S, Mohammed H, Shah NP (2016) Lymphocytosis after treatment with dasatinib in chronic myeloid leukemia: effects on response and toxicity. Cancer 122(9):1398–1407
Colom-Fernández B, Kreutzman A, Marcos-Jiménez A, García-Gutiérrez V, Cuesta-Mateos C, Portero-Sainz I, Pérez-García Y, Casado LF, Sánchez-Guijo F, Martínez-López J, Ayala RM, Boqué C, Xicoy B, Montero I, Soto C, Paz R, Silva G, Vega-Piris L, Steegmann JL, Muñoz-Calleja C (2019) Immediate effects of dasatinib on the migration and redistribution of naïve and memory lymphocytes associated with lymphocytosis in chronic myeloid leukemia patients. Front Pharmacol 10:1340
Mustjoki S, Ekblom M, Arstila TP, Dybedal I, Epling-Burnette PK, Guilhot F, Hjorth-Hansen H, Höglund M, Kovanen P, Laurinolli T, Liesveld J, Paquette R, Pinilla-Ibarz J, Rauhala A, Shah N, Simonsson B, Sinisalo M, Steegmann JL, Stenke L, Porkka K (2009) Clonal expansion of T/NK-cells during tyrosine kinase inhibitor dasatinib therapy. Leukemia 23(8):1398–1405
Hochhaus A, Baccarani M, Silver RT, Schiffer C, Apperley JF, Cervantes F, Clark RE, Cortes JE, Deininger MW, Guilhot F, Hjorth-Hansen H, Hughes TP, Janssen JJWM, Kantarjian HM, Kim DW, Larson RA, Lipton JH, Mahon FX, Mayer J, Nicolini F, Niederwieser D, Pane F, Radich JP, Rea D, Richter J, Rosti G, Rousselot P, Saglio G, Saußele S, Soverini S, Steegmann JL, Turkina A, Zaritskey A, Hehlmann R (2020) European LeukemiaNet 2020 recommendations for treating chronic myeloid leukemia. Leukemia 34(4):966–984
Pilalas D, Koletsa T, Arsos G, Panselinas G, Exadaktylou P, Polychronopoulos G, Savopoulos C, Kaiafa GD (2020) Dasatinib associated lymphadenopathy in a chronic myeloid leukemia patient: a case report. Medicine 99(45):e22791
Ozawa MG, Ewalt MD, Grantzinger D (2015) An underrecognized entity with characteristic morphology. Am J Surg Pathol 39:1363–1369
Bouquet E, Jourdain A, Machet MC, Beau-Salinas F, Jonville-Béra AP (2017) Dasatinib-associated follicular lymphoid hyperplasia: first pediatric case report and literature review. Pediatr Blood Cancer 64(11)
Rawstron AC (2010) Monoclonal B-cell lymphocytosis. 36th National Hematology Congress, November 3, 163–6
Iurlo A, Bucelli C, Cattaneo D, Orofino N, Giannotta JA, Zappa M, Gianelli U, Cortelezzi A (2017) Reactive follicular hyperplasia on dasatinib treatment for chronic myeloid leukemia. Ann Hematol 96(11):1953–1954
Kurosaki T, Hikida M (2009) Tyrosine kinases and their substrates in B lymphocytes. Immunol Rev 228(1):132–148
Li HL, Davis WW, Whiteman EL, Birnbaum MJ, Puré E (1999) The tyrosine kinases Syk and Lyn exert opposing effects on the activation of protein kinase Akt/PKB in B lymphocytes. Proc Natl Acad Sci USA 96(12):6890–6895
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Solmaz, A.A., Ozcan, H.E., Ugurludogan, A.C. et al. Reversible follicular lymphoid hyperplasia related with dasatinib: first case report from Turkey. J Hematopathol 15, 179–183 (2022). https://doi.org/10.1007/s12308-022-00498-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12308-022-00498-4