Abstract
RNA interference is a powerful tool for the functional analysis of proteins by specific gene knockdown. In this study, we devised a rapid and efficient way to screen suitable siRNA sequences and subsequently employ them for specific gene knockdown in usually hard-to-transfect lymphoid cell lines, using a self-inactivating lentiviral vector. Two proteins with different half-lives were chosen, cyclin D1 and STAT3. A specific lacZ reporter fusion assay was used to identify highly effective siRNA sequences. Only siRNA molecules with more than 85% of knockdown efficiency were selected for the generation of lentiviral transfer vectors. Transduction rates of 75–99% were achieved in the lymphoma cell lines Granta 519 (mantle cell lymphoma), Karpas 299, and SUDHL-1 (anaplastic large T cell lymphoma), as demonstrated by green fluorescent protein expression in fluorescence-activated cell sorting analysis. The high level of transduction efficiency allows RNA interference studies to be performed on transduced cells without further manipulation, such as cell sorting or cloning. The LacZ reporter system together with the lentivirus technology is a very important tool in the hematology field, which enables experiments in lymphoid cells that were not possible before.
Similar content being viewed by others
Introduction
Normal lymphocytes and primary tumors, as well as cell lines of the lymphoid lineage are known to be difficult to transfect efficiently. Previous attempts to transduce B and T lymphocytes using retroviruses [1], diethylaminoethyl dextran, or liposomes [2] have had limited success, resulting in transfection efficiencies of only 10% to 30%. This relatively low transfection efficiency is a major obstacle for siRNA strategies to efficiently test their contribution to normal biological responses in lymphocyte populations without further manipulation and cell sorting. Lentiviruses are an exciting gene delivery tool because of their ability to efficiently transduce both dividing and nondividing target cell populations. Additionally, their capacity to establish long-lasting transgene expression due to chromosomal integration of the proviral DNA makes this technique very attractive in the lymphoma research field [3].
Intense interest in the field of RNAi (RNA interference) has facilitated its rapid transformation from a biological phenomenon to a valuable research tool used to silence target gene expression [4, 5]. RNAi can be mediated either by small interfering RNAs (siRNAs) of about 21 nucleotides or by stably expressed short hairpin RNAs (shRNAs), which are processed by Dicer into siRNAs [6, 7]. Two major problems are evident for studying the lymphocytes biology: first to achieve their high transfection rate and second to select the appropriate highly efficient siRNA sequence. The real challenge of RNAi technology is designing an effective siRNA sequence [8]. Any region of mRNA can be targeted; however, sequences that are known sites for mRNA-binding proteins in the 5′ untranslated region (UTR), 3′ UTR, start codon, or exon–exon boundaries should be avoided [9]. Many groups have demonstrated that the specific RNAi molecules targeting different regions of a transcript can vary widely in their effectiveness at inducing gene silencing [10, 11]. Some recently published reports advised that chemically synthesized siRNAs should be employed at low concentrations in order to prevent unspecific effects, thus underlining the necessity to select highly efficient siRNAs [12]. Not all rationally designed si/shRNAs will achieve knockdown of gene expression at the same level, and, therefore, it is very desirable to evaluate the silencing capability of several candidates before committing to the actual experiment.
Many of the proposed guidelines for specific siRNA design have been incorporated into a variety of public and commercial computational design tools following the recommendations originally described by Tuschl and coworkers [13, 14]. Although freely available conventional siRNA design programs speed the design process, researchers are still faced with the task of testing multiple siRNAs to identify a suitable potent siRNA molecule. Although siRNA-induced gene silencing is primarily an mRNA-level event, it is recommended that both mRNA and protein levels are analyzed. The advantage of analyzing the protein expression by Western blot is the possibility to quantitate the knockdown level of the endogenous protein of interest.
The aim of this study is to develop a protocol to achieve efficient transduction of highly efficient siRNA into B and T lymphoma cells using a lentiviral vector. For this purpose, we have chosen STAT3 and cyclin D1 proteins as targets because the expression of these proteins is associated with lymphomagenesis and cancer progression [15, 16], and they have different protein half-lives in the cells [16–18].
Materials and methods
Design of shRNA constructs and cloning into pSuper vector
Specific shRNAs were designed by using bioinformatics tools publicly available from Dharmacon, Invitrogen, Qiagen, or MWG websites. We designed seven different sequences for efficient cyclin D1 targeting by shRNA. Of the four Stat3 shRNAs analyzed, one has been published [19], and we designed three additional sequences for further detailed analysis. As a control, “control” siRNA (Dharmacon Research, Chicago, IL, USA), with the sense sequence 5′-GCCGCUUUGUAGGAUAGAG-3′ which lacks complementary sequences in the human genome, was used.
Oligonucleotides (listed in Table 1) were cloned into pSuper vector as described [6]. In brief, the double-stranded DNA templates encoding siRNA oligonucleotides for cyclin D1 or STAT3 were synthesized. The specific oligonucleotide sequence contained a sense strand of 19 nucleotides followed by a short spacer (TTCAAGAGA) and the reverse complement of the sense strand. Five thymidines were added at the end of synthesized oligonucleotide as RNA polymerase III transcriptional stop signal. Each pair of oligos was annealed at 20 μM in annealing buffer (100 mM potassium acetate, 30 mM HEPES–KOH (pH 7.4), 2 mM magnesium acetate) at 95°C for 4 min, followed by incubation at 70°C for 10 min and slow cooling to room temperature. Forty picomolars of annealed oligos were phosphorylated by T4 polynucleotide kinase before they were ligated into pSuper vector digested by BglII and HindIII. All constructs were checked by EcoRI–HindIII digestion and by sequencing.
Generating the lacZ-cyclin D1 (pLC1) and lacZ-STAT3 (pLS3) screening construct
Ultimate hORF entry clone containing the human cyclin D1 gene (Invitrogen, ORF no. IOH 1962) and human STAT3 gene (Invitrogen, ORF no. IOH 14348), respectively, were transferred into pSCREEN-iT/lacZ-DEST vector using the LR recombination reaction with specific enzyme LR Clonase, characteristic of gateway cloning (Invitrogen, Carlsbad, CA, USA). Cloning used in this study allows in vitro recombination-based transfer of DNA from one vector to another without the use of restriction enzymes. Briefly, 150 ng of destination vector (LacZ-DEST) and 50–150 ng of entry clone (IOH 1962 or IOH 14348) were mixed in TE buffer (pH 8.0). LR Clonase enzyme mix (4 μl) was added and incubated for 1 h at room temperature. Reaction was stopped with 2 μg of proteinase K solution for 10 min at 37°C. Part of LR reaction (3 μl) was transformed into TOP10 Escherichia-coli-competent cells (Invitrogen, Carlsbad, CA, USA) and positive clones were selected on LB plates containing 100 μg/ml ampicillin (Sigma, St. Louis, MO, USA). This way, we generated the lacZ-STAT3 (pLS3) and the lacZ-cyclin D1 (pLC1) in frame fusion screening constructs.
β-galactosidase assay
Simultaneous delivery of the shRNA constructs with the pLS3 or pLC1 plasmid induces cleavage of the lacZ fusion transcript, resulting in reduction of β-galactosidase activity. The activity of β-galactosidase is directly dependent on the mRNA stability of the fusion protein. The β-galactosidase assay was performed using the FluoReporter LacZ/Galactosidase Quantitation kit according to the manufacturer’s instructions (Invitrogen, Carlsbad, CA, USA). This kit contains a fluorogenic 3-carboxy-umbelliferyl β-D-galactopyranoside (CUG) substrate from Molecular Probes (cat. no. F-2905) that allows highly sensitive measurement of β-galactosidase activity. Briefly, 20 μl of cell lysates or 10−1 or 10−2 dilutions were used for measurements in black-walled clear-bottom 96-well microtiter plates (Costar, Corning, NY, USA) containing 100 μl of the 1.1-mM CUG substrate working solution. The reactions were incubated for 10–30 min at room temperature and stopped by adding 50 μl of stop solution (0.2 M Na2CO3). Fluorescence emission of the CUG substrate was measured using a fluorescence plate reader (Fluoroskan Ascent CF reader, Thermo Scientific, Waltham, MA, USA) equipped with the proper filter set (excitation filter centered at ∼390 nm and emission filter centered at ∼460 nm).
Cell culture and transfections
The lymphoma cell lines Granta 519 (mantle cell lymphoma), Karpas 299, and SUDHL-1 (anaplastic large cell lymphoma) were cultured in RPMI 1640 supplemented with 10% fetal bovine serum (FCS), 2 mM glutamine, 100 μg/ml penicillin, and 100 μg/ml streptomycin (Life Technologies, Inc., Gaithersburg, MD, USA). The human embryonic kidney 293 T cells were grown in Dulbecco’s modified eagles medium with 10% FCS, 100 μg/ml penicillin, and 100 μg/ml streptomycin (Invitrogen, Carlsbad, CA, USA). Transfections were performed with Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. Typically, cells growing on 60-mm Petri dishes were transfected with 0.5 μg reporter plasmid (pLC1 or pLS3) and 5.0 μg pSuper empty vector or pSuper with a specific siRNA sequence (shRNA vector). Cells were analyzed 48 or 72 h after transfection. To estimate the transfection efficiency, a green fluorescent protein (GFP)-coding plasmid (pEGFP, Clontech, Mountain View, CA, USA) was transfected in 293 T cells and the number of GFP-expressing cells was counted by fluorescence microscopy (Apotome, Zeiss MicroImaging GmbH, Göttingen, Germany). Transfection efficiencies for most experiments ranged from 70% to 99%.
Virus production and concentration
The pFUGW transfer vector, the corresponding packaging plasmid, and the G-protein of vesicular stomatitis virus envelope plasmid were a kind gift of Dr. Baltimore [3]. The pFUGW lentiviral vector allows the expression of EGFP reporter gene driven by an internal ubiquitin-C promoter. To insert a specific shRNA into the lentiviral vector, shRNA together with human H1 promoter from pSuper constructs was digested with SmaI and HincII and ligated into pFUGW digested with PacI, followed by blunting using T4 DNA polymerase. The orientations of the fragments were confirmed by ClaI and EcoRI digestion. As controls, pFUGW derivative vectors containing only an H1 promoter from pSuper plasmid or the control shRNA sequence under H1 promoter were generated and named “empty virus” and “control shRNA,” respectively.
Replication-defective lentiviral virions were produced by transient cotransfection of 7.5 μg pCMVdeltaR8.9, 5 μg pHCMV-G, and 10 μg of the pFUGW vector or its derivatives into 293 T cells with the Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) transfection system. The media was changed 6 h after transfection and supernatant containing the virus was harvested 48 h after transfection, cleared of debris by low-speed centrifugation, and filtered through 0.45-μm Stericup filters (Millipore, Billerica, MA, USA). Yields were typically 80 to 120 ml from a ten to 15 10-cm dishes. The lentivirus was concentrated by ultrafiltration using Amicon-20 100-kDa-molecular-weight cut-off columns (Millipore, Billerica, MA, USA) in accordance to the manufacturer’s guidelines. The virus was subsequently aliquoted (100 μl) and supernatants were stored at −80°C. Virus titers (multiplicity of infection—MOI) were determined by fluorescence-activated cell sorting (FACS) analysis of a known number of 293 T cells infected with serially diluted viral supernatant and GFP expression analysis.
Viral infections of suspension lymphoma cell lines
Granta 519, Karpas 299, and SUDHL-1 cells (2 × 106 per milliliter) were resuspended in lentivirus-containing supernatant in the presence of polybrene (8 μg/ml) in a six-well plate. Plates were centrifuged at 1,000 g for 90 min. After centrifugation, cells were washed, resuspended in fresh culture medium, and incubated at 37°C in a CO2 incubator for indicated time points.
Cytofluorimetric analysis (FACS) of infected cells
Three days after infection, cells were washed in phosphate-buffered saline (PBS) and resuspended in FACS buffer (PBS with 5% FCS and 1 μg/ml of propidium iodide—PI). PI was used to determine cell viability of infected cells and uninfected control. Gene transduction efficiency was determined by cytofluorimetric analysis using the BD FACSCalibur™ flow cytometer (BD Biosciences, San Jose, CA, USA) and analyzed with BD CellQuest Pro™ software. Infected cells were detected on the basis of GFP fluorescence relative to uninfected control.
Western blot analysis
Cells were washed in PBS, pelleted, and lysed, as described [20]. The immunoreagents used for Western blot were rabbit polyclonal antibody against cyclin D1 (H-295; Santa Cruz Biotechnology, Sc-753) and mouse monoclonal anti-STAT3 antibody (Transduction Laboratories, cat. no. 610190). Mouse monoclonal antitubulin antibody (Sigma, Clone B512, cat. no. T-5168) was used as loading control. All experiments were repeated several times and gave similar results.
Real-time quantitative RT-PCR
Real-time quantitative reverse-transcription polymerase chain reaction (RT-PCR) analysis was performed with the ABI Prism 7500 Sequence Detection System (Applied Biosystems, Foster City, CA, USA). For the quantification of cyclin D1 and STAT3, we used gene expression assays from Applied Biosystems (cyclin D1, Hs00277039_m1; STAT3, Hs01047572_m1). TATA box-binding protein (TBP) was used as the housekeeping gene, as previously described [16]. Target gene expression was normalized to TBP and the target gene expression was analyzed by the \(2^{ - \Delta \Delta \operatorname{CT} } \) formula. All reactions were performed at least twice in duplicate.
Results
Determination of knockdown efficiency for STAT3 shRNA expression vectors
Specific fusion protein lacZ-STAT3 (pLS3) was first constructed (described in “Materials and methods”) to precisely quantify the shRNA effect on STAT3 expression. The β-galactosidase activity was normalized to 100% with the activity of the fusion protein in cell extracts from 293 T cells transfected only with lacZ-STAT3 fusion (pLS3; Fig. 1a). The knockdown efficiency of four STAT3 shRNA constructs was investigated. All four designed sequences for STAT3 knockdown were effective on expression of lacZ-STAT3 fusion (Fig. 1a), showing striking reduction in β-galactosidase activity. The best knockdown effect was achieved with the constructs pS-Gh1 (97%) and pS-Dh353 (98%), whereas the other two constructs pS-INV1 and pS-Dh17 showed moderate activity with 80% and 75% knockdown efficiency, respectively. In order to see whether the effect was specific, cotransfection of cyclin D1 shRNA–pSuper expression vector (pS-Dh2) with pLS3 was performed. pS-Dh2 showed induction of lacZ-STAT3 fusion activity up to 195%, possibly due to the presence of a negative feedback loop between cyclin D1 and STAT3.
shRNAs against STAT3 specifically silence lacZ-STAT3 reporter expression as well as endogenously expressed STAT3 in a 293-T-cell cotransfection model. a Simultaneous delivery of different STAT3 shRNAs with the lacZ-STAT3 fusion plasmid induces reduction of β-galactosidase activity. β-galactosidase activity is normalized to 100% in 293 T cells transfected only with specific LacZ fusion. pSuper represents empty vector control. Cells were analyzed 48 h after transfection. b Western blot for STAT3 protein of cells transfected with lacZ-STAT3 reporter (pLS3) and simultaneously with control plasmid (pSuper) or different shRNA plasmids (pS-Gh1, pS-Inv1, pS-Dh353, and pS-Dh17) 48 h and c 72 h after transfection. In contrast to the fusion protein, endogenous STAT3 disappears only at 72 h after transfection. The membranes were reprobed with antitubulin to confirm equal loading
The knockdown effect with the specific STAT3 shRNAs was corroborated by Western blot analysis of cell extracts analyzed 48 h after cotransfection of shRNA expression plasmids with the fusion plasmid (pLS3). The fusion protein showed a specific protein band of 210 kDa (Fig. 1b). However, the endogenously expressed STAT3 protein (92 kDa) was not downregulated. Since the half-life of STAT3 is approximately 72 h, we repeated the experiment and analyzed the cell extracts of the 293-T-cell line 72 h after cotransfection with the suitable shRNA constructs. This time, a complete knockdown with shRNA-Gh1 was demonstrated for both the fusion (210 kDa) protein and the endogenously expressed STAT3 protein (92 kDa) by Western blot (Fig. 1c).
Determination of knockdown efficiency for cyclin D1 shRNA expression vectors
Seven H1 RNA promoter-mediated shRNA expression vectors to target cyclin D1 mRNA were constructed. The bioinformatics tools used for design were from MWG (pS-M2 construct), Dharmacon (pS-Dh1, pS-Dh2, and pS-Dh3), and Invitrogen (pS-SA1, pS-AS1, and pS-GA1 constructs). All constructs have a 19-bp-long specific sense sequence, except pS-GA1, which has 21 bp (it has the same 19 bp like pS-SA1 plus two extra base pairs up to 21; see Table 1). Constructs pS-Dh1, pS-Dh2, and pS-Dh3 were designed in the same coding region of cyclin D1 and have 1- or 2-bp differences (see alignment below).

With these seven oligos for cyclin D1, we tried to cover the main regions for efficient siRNA silencing proposed by different bioinformatics web tools.
To precisely quantify the shRNA effect on cyclin D1, specific fusion protein lacZ-cyclin D1 (pLC1, described in “Materials and methods”) was constructed and transfected into the 293-T-cell line. Cotransfection of pSuper (pS) empty vector or control (pSS) shRNA sequence with pLC1 did not change the expression of the fusion protein significantly. Constructs pS-Dh1, pS-Dh3, and pS-SA1 were characterized as moderately active molecules with a knockdown efficiency of 80%. Construct pS-Dh2 was characterized as highly active shRNA with 96% of knockdown efficiency. The other shRNAs failed to show significant activity. Construct pS-Gh1, with the best knockdown efficiency for STAT3, was used as an independent control for specificity of the lacZ-cyclin D1 fusion knockdown. Cotransfection of STAT3 shRNA-Gh1 with fusion plasmid (pLC1) showed no effect on lacZ-cyclin D1 expression in the 293-T-cell line (Fig. 2a).
shRNAs against cyclin D1 are able to specifically silence lacZ-cyclin D1 reporter expression and endogenously expressed cyclin D1. a Cotransfection of different shRNA constructs for cyclin D1 with lacZ-cyclin D1 reporter fusion (pLC1). shRNA effect was measured 48 h after transfection by β-galactosidase assay. pSuper and pS control represent controls with empty vector and pSuper with control shRNA sequence, respectively. STAT3 shRNA-Gh1 was used as an irrelevant control for lacZ-cyclin D1 reporter expression. b Western blot for cyclin D1 demonstrates striking reduction of both endogenous cyclin D1 as well as fusion protein in the cells cotransfected with specific shRNA constructs (pS-Dh2 and pS-SA1) 48 h after transfection. The same membrane was reprobed with anti-tubulin
The relationship between the knockdown effect on cyclin D1 protein and β-galactosidase assay was analyzed by Western blot from 293-T-cell extracts prepared 48 h after transfection either with lacZ-cyclin D1 fusion (pLC1) alone or cotransfected with specific shRNA constructs (Fig. 2b). The strongest reduction in β-galactosidase activity was induced by pS-Dh2, followed by pS-SA1 construct (Fig. 2a), which showed an excellent correlation with the Western blot results. Both the fusion protein (156 kDa) and the endogenous cyclin D1 (36 kDa) were completely downregulated by pS-Dh2, whereas pS-SA1 showed strong but not complete downregulation of the fusion protein.
Sensitivity and reproducibility of the β-galactosidase assay
To determine the sensitivity of the method in more detail, we used different ratios of reporter plasmid pLC1 in cotransfection with the shRNA vectors. Figure 3 shows the effect of shRNA constructs when 100, 500 ng, or 1 μg of reporter plasmid (pLC1) were used. The best ratio to compare and to find the best shRNA with the highest efficiency is 500 ng of reporter with 5 μg of shRNA vector (ratio 1:10). Vector pS-Dh2 was highly active with all analyzed concentrations of reporter, indicating that it is the most efficient construct. The efficient downregulation of the target protein with the same shRNA was achieved independent of protein expression level in the cell, as evidenced by comparison of knockdown in 293 T cells with endogenous expression of cyclin D1 and lacZ-cyclin D1 fusion expression (Fig. 2b).
Sensitivity and reproducibility of β-galactosidase assay. Different ratios of different shRNA plasmids and reporter fusion (pLC1) in cotransfection were employed. Concentration of shRNA plasmids for cotransfection was constant (5 μg) and pLC1 was 100 ng (light gray boxes), 500 ng (gray boxes), and 1 μg (dark gray boxes). Cells were analyzed 48 h after transfection
Efficient transfer of cyclin D1 shRNA in B cell lymphoma cells using lentiviral vector
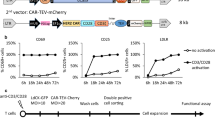
Since lentiviruses can infect a wide range of dividing and nondividing cells, we tested whether the lentiviral vector pFUGW can be used for siRNA-mediated gene silencing in human lymphoma cell lines. The most efficient construct in β-galactosidase assay for cyclin D1 (Fig. 3), pS-Dh2, was used for lentivirus transfer vector production. The resulting lentiviral vector pF-Dh2 was packaged and first titrated on 293 T cells for determination of MOI. To identify the titer required for efficient transduction, the mantle cell lymphoma cell line Granta 519 was transduced with increasing MOI (15, 45, and 90; Fig. 4a–c). Although the viability (65%) and the transduction efficiency was equally good with the different MOI, the amount of shRNA in the cells was increasingly higher with 45 and 90 MOI, as judged by the intensity of GFP in the FACS analysis (Fig. 4a). Western blot analysis showed an excellent correlation between cyclin D1 protein expression and the amount of MOI used, with practically complete knockdown of cyclin D1 with 90 MOI (Fig. 4b). However, quantitative RT-PCR of cyclin D1 mRNA demonstrated similar cyclin D1 levels between 45 and 90 MOI, whereas, with 90 MOI nonspecific downregulation of cyclin D1 mRNA was observed in the controls (Fig. 4c). Therefore, 40–45 MOI were used for all subsequent experiments in lymphoma cell lines. In Granta 519 cells and corresponding controls, high levels (98% on average) of GFP were expressed 3 days after transduction, with specific cyclin D1 downregulation as shown in Fig. 4b.
Successful infection of B cell lymphoma cells (Granta 519) with lentivirus. a The efficiency of viral infection was analyzed, detecting GFP expression by FACS. Granta 519 control cells and infected cells were 65% viable 3 days after infection and infection ranged between 93.2% and 98.9%, dependent of MOI (15, 45, 90) used for transduction. b Specific knockdown effect for cyclin D1 was shown by Western blot from cell extracts prepared 3 days after infection with different MOI, for corresponding controls and cyclin D1 shRNA-Dh2. c qRT-PCR analysis of cyclin D1 mRNA was performed relative to the TBP housekeeping gene. Results are depicted as mRNA concentration relative to the control uninfected cells (Granta 519-c). Data were analyzed according to the ΔCT method
Effective gene silencing of STAT3 protein in T-cell lymphoma cells using lentiviral vectors
The most efficient construct in β-galactosidase assay for STAT3 (Fig. 1), pS-Gh1, was used for lentivirus transfer vector production (pF-Gh1). ALK+ anaplastic large T cell lymphoma cells (Karpas 299 and SUDHL-1) were exposed to supernatant containing lentiviral particles at 40 MOI in one round of infection. Karpas 299 control and infected cells were 93% viable 3 days after infection and on average 80% infected (Fig. 5a). SUDHL-1 control and infected cells were 43% viable 3 days after infection and 99% infected (Fig. 5b). The level of knockdown effect for STAT3 was analyzed by Western blot from cell extracts prepared 3 days after infection (Fig. 5c). The specific downregulation of STAT3 protein correlated with the amount of transfected cells. Karpas 299, which showed around 80% transduction, had a strong reduction of STAT3 protein, whereas SUDHL-1 cells with 99% of viral infection showed complete inhibition of STAT3 protein. None of the cell lines showed side effects in the controls. To evaluate the stability of the lentivirus infection and siRNA-mediated gene silencing, we analyzed the GFP expression at several time points after gene transduction of Granta 519 and Karpas 299 cells. The percentage and the mean fluorescence intensity of GFP-positive cells were maintained over the time course, and importantly the GFP-positivity was also maintained even after freezing and thawing the cells (data not shown).
Successful infection of T cell lymphoma cells (Karpas 299 and SUDHL-1) with lentivirus. The efficiency of viral infection was analyzed, detecting GFP expression by FACS. a Karpas 299 noninfected and infected cells were 90% viable 3 days after infection and on average 75% infected. b SUDHL-1 control and infected cells were 43% viable 3 days after infection and 99% infected. c The level of knockdown effect for STAT3 was analyzed by Western blot from cell extracts prepared 3 days after infection with corresponding controls and STAT3 shRNA-Gh1. The specific downregulation of STAT3 protein was shown in the infected cells and no side effects were seen when membrane was probed with anti-ALK1. The same membrane was probed with anti-tubulin. d qRT-PCR analysis of STAT3 mRNA was performed relative to the TBP housekeeping gene. Results are depicted as mRNA concentration relative to Karpas 299 (light gray boxes) and SUDHL-1 (gray boxes) control cells. Data were analyzed according to the ΔCT method
Discussion
In this study, we have successfully used a combination of two techniques to achieve efficient transduction of specific shRNA with high knockdown efficiency into B and T lymphoma cells, with infection rates in the range of 80% to 99%. The first technique is a highly sensitive β-galactosidase reporter assay based on a specific lacZ reporter fusion with the corresponding gene of interest that enables fast and easy recognition of the most efficient siRNA sequence for target knockdown. The second technique requires RNAi molecules with more than 85% of knockdown efficiency that are further cloned into a lentiviral vector (pFUGW) [3] for virus production and successful transduction of lymphoma cell lines.
The need for a strong specific gene silencing effect by RNAi means there is an absolute requirement to test the specificity and efficiency of siRNAs before embarking on phenotype analysis [8, 21]. Although it is expected that chemically synthesized siRNAs and corresponding hairpin-based shRNAs should have the same gene knockdown effect, it has been shown that si/shRNAs exhibit similar but not identical sequence preferences [22]. With the approach described here, the generation of constructs is straightforward, relying on the use of synthetic oligonucleotides and a single restriction site that ensures directional cloning. The sensitivity of the LacZ reporter fusion system allows the identification of RNAi molecules that are the most potent in inducing target gene knockdown even at protein level. In this study, two proteins that have been recognized to be important in lymphomagenesis were chosen, cyclin D1 with a short half-life and STAT3 with a long half-life. In both instances, efficient knockdown activity of the fusion protein with specific shRNA was achieved. Since no preexisting pool of the LacZ fusion protein is available in the 293 T cells, the protein half-life to evaluate the shRNA efficiency is not an issue. Using this validation approach, it is very simple to identify the shRNAs that are most effective at knocking down the target gene. Nevertheless, when specific shRNA is used for knockdown of endogenous proteins, the half-life of the protein has to be taken in consideration to decide the right time of the experiment. This was evidenced in this study by STAT3 protein, where a big difference in knockdown efficiency was seen after 48 or 72 h, respectively. In addition, we demonstrated that assessment of shRNAs is not influenced by the amount of the reporter construct used for analysis since the most effective shRNA is reliably identified after cotransfection with both low (100 ng) and high (1 μg) concentration of the fusion gene.
For successful inhibition of target genes, the efficiency of the shRNA molecules and infection rate of the target cells are the two critical parameters. On one side, only shRNA molecules with more than 85% of knockdown efficiency (highly efficient) should be considered to further generate lentiviral transfer vectors and complex lentivirus production. On the other side, the optimal MOI should be established for each cell population to be analyzed [23, 24]. Our data describe the conditions of infection required for optimal gene transduction for lymphoid cell lines, which may have to be modified for other types of target cells. The limiting factor for high transduction rate and the main difference between viral infection of adherent cells and suspension cells is the centrifugation step, during which viral infection occurs in the latter. Since there is less contact between viral particles and suspension cells as compared to adherent cells, higher infection units per cell are needed to obtain efficient transduction. The fraction of infected cells rises with increasing MOI and reaches a plateau between 35 and 45 MOI. This is the optimal point where the highest levels of gene knockdown are achieved, with a minimum of nonspecific effects. However, we have encountered lymphoma cell lines where much higher MOI (up to 80) are needed to achieve around 80% of cell transduction (data not shown). The lentiviral vectors have the advantage to remain stable within the cell for long periods of time [25]. The monitoring of GFP expression by FACS analysis not only gives information about the amount of transduced cells but also about the stability of the transduction. GFP expression was analyzed up to 12 weeks, and its expression remained stable even after cell freezing and thawing.
In summary, we describe the use of the lentiviral vector system to obtain highly efficient and long-term repression of gene expression in lymphoid cell lines, which represents a major improvement over previously published methods [1, 2, 25]. The high ability of lentivirus to transduce lymphoma cells and to achieve high knockdown effect through RNAi opens new avenues for genetic characterization and provides a useful tool for basic molecular studies of lymphoma cell biology and therapy regimens. The high level of transduction efficiency (85% on average) will allow RNA interference studies to be performed on transduced populations of cells without further manipulation such as cell sorting or cloning.
References
Guven H, Konstantinidis KV, Alici E et al (2005) Efficient gene transfer into primary human natural killer cells by retroviral transduction. Exp Hematol 33(11):1320–1328
Huang H, Pannetier C, Hu-Li J et al (1998) Transient transfection of primary T helper cells by particle-mediated gene transfer. J Immunol Methods 215(1–2):173–177
Lois C, Hong EJ, Pease S et al (2002) Germ line transmission and tissue-specific expression of transgenes delivered by lentiviral vectors. Science 295(5556):868–872
Sledz CA, Williams BR (2004) RNA interference and double-stranded-RNA-activated pathways. Biochem Soc Trans 32(Pt 6):952–956
Sledz CA, Holko M, de Veer MJ et al (2003) Activation of the interferon system by short-interfering RNAs. Nat Cell Biol 5(9):834–839
Brummelkamp TR, Bernards R, Agami R (2002) A system for stable expression of short interfering RNAs in mammalian cells. Science 296(5567):550–553
Paddison PJ, Caudy AA, Bernstein E et al (2002) Short hairpin RNAs (shRNAs) induce sequence-specific silencing in mammalian cells. Genes Dev 16(8):948–958
Sledz CA, Williams BR (2005) RNA interference in biology and disease. Blood 106(3):787–794
Dykxhoorn DM, Novina CD, Sharp PA (2003) Killing the messenger: short RNAs that silence gene expression. Nat Rev Mol Cell Biol 4(6):457–467
Bohula EA, Salisbury AJ, Sohail M et al (2003) The efficacy of small interfering RNAs targeted to the type 1 insulin-like growth factor receptor (IGF1R) is influenced by secondary structure in the IGF1R transcript. J Biol Chem 278(18):15991–15997
Vickers TA, Koo S, Bennett CF et al (2003) Efficient reduction of target RNAs by small interfering RNA and RNase H-dependent antisense agents. A comparative analysis. J Biol Chem 278(9):7108–7118
Schubert S, Grunweller A, Erdmann VA et al (2005) Local RNA target structure influences siRNA efficacy: systematic analysis of intentionally designed binding regions. J Mol Biol 348(4):883–893
Elbashir SM, Harborth J, Lendeckel W et al (2001) Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature 411(6836):494–498
Elbashir SM, Lendeckel W, Tuschl T (2001) RNA interference is mediated by 21- and 22-nucleotide RNAs. Genes Dev 15(2):188–200
Quintanilla-Martinez L, Kremer M, Specht K et al (2003) Analysis of signal transducer and activator of transcription 3 (Stat 3) pathway in multiple myeloma: Stat 3 activation and cyclin D1 dysregulation are mutually exclusive events. Am J Pathol 162(5):1449–1461
Specht K, Haralambieva E, Bink K et al (2004) Different mechanisms of cyclin D1 overexpression in multiple myeloma revealed by fluorescence in situ hybridization and quantitative analysis of mRNA levels. Blood 104(4):1120–1126
Chiarle R, Simmons WJ, Cai H et al (2005) Stat3 is required for ALK-mediated lymphomagenesis and provides a possible therapeutic target. Nat Med 11(6):623–629
Diehl JA, Zindy F, Sherr CJ (1997) Inhibition of cyclin D1 phosphorylation on threonine-286 prevents its rapid degradation via the ubiquitin–proteasome pathway. Genes Dev 11(8):957–972
Gao LF, Xu DQ, Wen LJ et al (2005) Inhibition of STAT3 expression by siRNA suppresses growth and induces apoptosis in laryngeal cancer cells. Acta Pharmacol Sin 26(3):377–383
Quintanilla-Martinez L, Pittaluga S, Miething C et al (2006) NPM-ALK-dependent expression of the transcription factor CCAAT/enhancer binding protein beta in ALK-positive anaplastic large cell lymphoma. Blood 108(6):2029–2036
Shao Y, Chan CY, Maliyekkel A et al (2007) Effect of target secondary structure on RNAi efficiency. RNA 13(10):1631–1640
Li L, Lin X, Khvorova A et al (2007) Defining the optimal parameters for hairpin-based knockdown constructs. RNA 13(10):1765–1774
Logan AC, Nightingale SJ, Haas DL et al (2004) Factors influencing the titer and infectivity of lentiviral vectors. Hum Gene Ther 15(10):976–988
Fish RJ, Kruithof EK (2004) Short-term cytotoxic effects and long-term instability of RNAi delivered using lentiviral vectors. BMC Mol Biol 5:9
Piva R, Chiarle R, Manazza AD et al (2006) Ablation of oncogenic ALK is a viable therapeutic approach for anaplastic large-cell lymphomas. Blood 107(2):689–697
Acknowledgements
We are grateful to Dr. David Baltimore and to Dr. Abel Sánchez-Aguilera for donating pFUGW vector and packaging plasmids for lentivirus production. We are grateful to Mark Raffeld, MD, for donating some of the cell lines used in this study. The authors thank Dr. Ludger Hengst for donating pSuper and pS-Scramble-Control vectors and Ulrike Reich for excellent technical assistance. The authors also thank to Dr. Tim Schröder and Mrs. Andrea Hermann for assistance with FACS analysis.
Supported in part by a grant from the Deutsche Forschungsgemeinschaft (FE 597/3-1) to L.Q-M. and F.F. and the Mantle Cell Consortium of the Leukemia Research Foundation to L.Q-M.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary materials
Below is the link to the electronic supplementary materials.
Supplemental Fig. 1
(DOC 343 KB)
Supplemental Fig. 2
(DOC 343 KB)
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License ( https://creativecommons.org/licenses/by-nc/2.0 ), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Anastasov, N., Klier, M., Koch, I. et al. Efficient shRNA delivery into B and T lymphoma cells using lentiviral vector-mediated transfer. J Hematopathol 2, 9–19 (2009). https://doi.org/10.1007/s12308-008-0020-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12308-008-0020-x