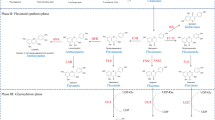
Abstract
In the present experiment a pteridophytic species Azolla and an angiospermic species Vernonia were evaluated on the basis of cellular reactivity for herbicidal action through ongoing concentrations. Initially, both the species recorded a significant activity of IAA-oxidase as mark of IAA metabolism with herbicidal sensitivity. Still, Vernonia species were more affected on 2,4-D mediated auxin catabolism. The loss of auxin concentrations on the tissues by 2,4-D reaction was also reflected on growth parameters including relative growth rate and chlorophyll biosynthesis. In a dose dependent manner Vernonia plants were more affected with loss of chlorophyll content and decline in relative growth rate. On the other hand, both those parameters were adjusted significantly with 2,4-D accumulation in Azolla. The stability of cellular metabolism was documented by significant down regulation of protein and lipid peroxidation with concomitant moderation to superoxide and hydrogen peroxide accumulation. The later two were more vulnerable to damage in the Vernonia plant with profuse accumulation of protein and lipid peroxidation products. Similarly, tissue specific reaction to superoxide and hydrogen peroxide accumulation were distinctly demarcated in two species significantly. As a whole, the cellular responses and metabolite distribution to 2,4-D sensitization are the features to describe bio-indices for aquatic fern species Azolla with comparison to angiospermic species Vernonia.












Similar content being viewed by others
Abbreviations
- IAA:
-
Indole-3-acetic acid
- 2,4-D:
-
2,4-dichloro-phenoxy acetic acid
- ROS:
-
Reactive oxygen species
- O2 − :
-
Superoxide radical
- H2O2 :
-
Hydrogen peroxide
- RH:
-
Relative humidity
- TCA:
-
Trichloro acetic acid
- NBT:
-
Nitro blue tetrazolium
- DAB:
-
Di-amino benzidine
- KI:
-
Potassium iodide
- MDA:
-
Malondialdehyde
- DNPH:
-
2,4 Dinitrophenylhydragene
- RWC:
-
Relative water content
References
Achary VMM, Jena S, Panda K, Panda B (2007) Aluminium induced oxidative stress and DNA damage in root cells of Allium cepa L. Ecotoxicol Environ Saf 70:300–310
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water stress studies. Plant Soil 39:205–207
Bradford MM (1976) Rapid and sensitive method for quantization of micro gram quantities of protein utilizing the principle of protein binding dye. Ann Biochem 72:248–254
Brazier M, Cole DJ, Edwards R (2002) O-glucosyltransferase activities toward phenolic natural products and xenobiotics in wheat and herbicide-resistant and herbicidesusceptible black-grass (Alopecurus myosuroides). Phytochemistry 59:149–156
Csiszar J, Szabo M, Erdei L, Marton L, Horvath F, Tari I (2004) Auxin autotrophic tobacco callus tissues resist oxidative stress: the importance of glutathione S-transferase and glutathione peroxidase activities in auxin heterotrophic and autotrophic calli. J Plant Physiol 161:691–699. doi:10.1078/0176-1617-01071
Das K, Mandal C, Ghosh N, Dey N, Adak MK (2013) Cadmium accumulation in Marsilea minuta Linn. and its antioxidative responses. Am J Plant Sci 4:365–371. doi:10.4236/ajps.2013.42A048
Dawood MJ (2016) Influence of osmoregulators on plant tolerance to water stress. Sci Agric 13(1):42–58
Dhindsa RS, Plumb-Dhindsa P, Thorpe TA (1981) Leaf senescence: correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J Exp Bot 32:93–101
Ebrahimzadeh H, Abrishamchi P (2001) Changes in IAA, phenolic compounds, peroxidase, IAA oxidase, and polyphenol oxidase in relation to flower formation in Crocus sativus. Rus J Plant Physiol 48(2):190–195
Cheng F, Cheng Z (2015) Research progress on the use of plant allellopathy in agriculture and the physiological and ecological mechanism of allellopathy. Front Plant Sci 6:1–16
Ghosh N, Adak MK, Ghosh PD, Gupta S, Sen Gupta DN, Mandal C (2011) Differential responses of two rice varieties to salt stress. Plant Biotechnol Rep 5:89–103
Gomez KA, Gomez AA (1984) Statistical procedures for agricultural research. Wiley, New York
Greenberg BM, Huang XD, Gerwing P, Yu XM, Chang P, Wu SS, Gerhardt K, Nykamp J, Lu X, Glick B (2008) Phytoremediation of salt impacted soils: greenhouse and the field trials of plant growth promoting rhizobacteria (PGPR) to improve plant growth and salt phytoaccumulation. In: Proceeding of the 33rd AMOP technical seminar on environmental contamination and response. Environment Canada, Ottawa, pp 627–637
Grossmann K, Kawiatkowski K, Tresh S (2001) Auxin herbicide induce H2O2 overproduction and tissue damage in cleavers (Galium aparine L). J Exp Bot 52(362):1811–1816
Hansen H, Grossmann K (2000) Auxin-induced ethylene triggers abscisic acid biosynthesis and growth inhibition. Plant Physiol 124(3):1437–1448
Hossain MA, Bhattacharjee S, Armin S-M et al (2015) Hydrogen peroxide priming modulates abiotic oxidative stress tolerance: insights from ROS detoxification and scavenging. Front Plant Sci 6:420. doi:10.3389/fpls.2015.00420
Jampeetong A, Brix H (2009) Effects of NaCl salinity on growth morphology, photosynthesis and proline accumulation of Salvinia natans. Aquat Bot 91:181–186
Mandal C, Ghosh N, Maiti S, Das K, Gupta S, Dey N (2013) Antioxidative responses of Salvinia (Salvinia natans Linn.) to aluminium stress and it’s modulation by polyamine. Physiol Mol Biol Plants 19:91–103
Mandal C, Ghosh N, Dey N, Adak MK (2014) Effects of putrescine on oxidative stress induced by hydrogen peroxide in Salvinia natans L. J Plant Interact. doi:10.1080/17429145.2013.871076
Mierziak J, Kostyn K, Kulma A (2014) Flavonoids as important molecules of plant interactions with the environment. Molecules 19(10):16240–16265
Mostofa MG, Rahman A, Ansary MU, Watanabe A, Fujita M, Tran LSP (2015) Hydrogen sulfide modulates cadmium-induced physiological and biochemical responses to alleviate cadmium toxicity in rice. Sci Rep 5:14078. doi:10.1038/srep14078
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Normanly J, Slovin J, Cohen J (2010) Auxin biosynthesis and metabolism. In: Davies P (ed) Plant hormones. Springer, Dordrecht, pp 36–62
Obidiegwu JE, Bryan GJ, Jones HG, Prashar A (2015) Coping with drought: stress and adaptive responses in potato and perspectives for improvement. Front Plant Sci 6:542. doi:10.3389/fpls.2015.00542
Oren BR, Tzin V, Tel-Or E, Zamski E (2004) Lead accumulation in the aquatic fern Azolla filiculoides. Plant Physiol Biochem 42:639–645. doi:10.1016/j.plaphy.2004.03.010
Parvaiz M (2014) Response of Maize to salt stress a critical review. Int J Healthc Sci (IJHS) 1(1):13–25
Porra RJ, Thompson WA, Kriedemann PE (1989) Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim Biophys Acta 975:384–394
Raghavan C, Ong EK, Dalling MJ, Stevenson TW (2005) Effect of herbicidal application of 2,4-dichlorophenoxyacetic acid in arabidopsis. Funct Integr Genom 5:4–17
Savitsky PA, Gazaryan IG, Tishkov VI, Lagrimini LM, Ruzgas T, Gorton L (1999) Oxidation of indole-3-acetic acid by dioxygen catalysed by plant peroxidases: specificity for the enzyme structure. Biochem J 340(Pt 3):579–583
Schroder R, Atkinson RG (2006) Kiwifruit cell walls: towards an understanding of softening. N Z J For Sci 36:112–129
Verbeke P, Siboska GE, Clark BFC, Rattan SIS (2000) Kinetin inhibits protein oxidation and glycoxidation in vitro. Biochem Biophys Res Comm 276:1265–1270
Wang J, Zhao FJ, Meharg AA, Raab A, Feldmann J, McGrath SP (2002) Mechanisms of arsenic hyper accumulation in Pteris vittata. Uptake kinetics, interactions with phosphate, and arsenic speciation. Plant Physiol 130(3):1552–1561. doi:10.1104/pp.008185
Weatherley PE (1965) The state and the movement of water in the leaf. Symp Soc Exp Biol 19:157–183
Weraduwage SM, Chen J, Anozie FC, Morales A, Weise SE, Sharkey TD (2015) The relationship between leaf area growth and biomass accumulation in Arabidopsis thaliana. Front Plant Sci 6:167. doi:10.3389/fpls.2015.00167
Acknowledgments
This work is financially supported by DST-PURSE programme, DST, Govt. of INDIA, New Delhi, for University of Kalyani.
Author information
Authors and Affiliations
Corresponding author
Additional information
Arnab Kumar De and Malay Kumar Adak contributed equally.
Rights and permissions
About this article
Cite this article
De, A.K., Dey, N. & Adak, M.K. Bio indices for 2,4-D sensitivity between two plant species: Azolla pinnata R.Br. and Vernonia cinerea L. with their cellular responses. Physiol Mol Biol Plants 22, 371–380 (2016). https://doi.org/10.1007/s12298-016-0375-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12298-016-0375-x