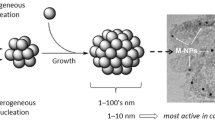
Abstract
Selective hydrogenation of acetylene in excess ethylene is an important reaction in both fundamental study and practical application. Pd-based catalysts with high intrinsic activity are commonly employed, but usually suffer from low selectivity. Pd single-atom catalysts (SACs) usually exhibit outstanding ethylene selectivity due to the weak π-bonding ethylene adsorption. However, the preparation of high-loading and stable Pd SACs is still confronted with a great challenge. In this work, we report a simple strategy to fabricate Pd SACs by means of reducing conventional supported Pd catalysts at suitable temperatures to selectively encapsulate the co-existed Pd nanoparticles (NPs)/clusters. This is based on our new finding that single atoms only manifest strong metal-support interaction (SMSI) at higher reduction temperature than that of NPs/clusters. The derived Pd SACs (Pd1/CeO2 and Pd1/α-Fe2O3) were applied to acetylene selective hydrogenation, exhibiting much improved ethylene selectivity and high stability. This work offers a promising way to develop stable Pd SACs easily.

Similar content being viewed by others
References
Borodziński, A.; Bond, G. C. Selective hydrogenation of ethyne in ethene-rich streams on palladium catalysts. Part 1. Effect of changes to the catalyst during reaction. Catal. Rev. 2006, 48, 91–144.
Borodziński, A.; Bond, G. C. Selective hydrogenation of ethyne in ethene-rich streams on palladium catalysts, Part 2: Steady-state kinetics and effects of palladium particle size, carbon monoxide, and promoters. Catal. Rev. 2008, 50, 379–469.
García-Mota, M.; Gómez-Díaz, J.; Novell-Leruth, G.; Vargas-Fuentes, C.; Bellarosa, L.; Bridier, B.; Pérez-Ramírez, J.; López, N. A density functional theory study of the “mythic” lindlar hydrogenation catalyst. Theor. Chem. Acc. 2011, 128, 663–673.
Teschner, D.; Borsodi, J.; Wootsch, A.; Revay, Z.; Havecker, M.; Knop-Gericke, A.; Jackson, S. D.; Schlogl, R. The roles of subsurface carbon and hydrogen in palladium-catalyzed alkyne hydrogenation. Science 2008, 320, 86–89.
Albani, D.; Shahrokhi, M.; Chen, Z. P.; Mitchell, S.; Hauert, R.; López, N.; Pérez-Ramírez, J. Selective ensembles in supported palladium sulfide nanoparticles for alkyne semi-hydrogenation. Nat. Commun. 2018, 9, 2634.
Zhou, H. R.; Yang, X. F.; Li, L.; Liu, X. Y.; Huang, Y. Q.; Pan, X. L.; Wang, A. Q.; Li, J.; Zhang, T. PdZn intermetallic nanostructure with Pd−Zn−Pd ensembles for highly active and chemoselective semi-hydrogenation of acetylene. ACS Catal. 2016, 6, 1054–1061.
Feng, Q. C.; Zhao, S.; Wang, Y.; Dong, J. C.; Chen, W. X.; He, D. S.; Wang, D. S.; Yang, J.; Zhu, Y. M.; Zhu, H. L. et al. Isolated single-atom Pd sites in intermetallic nanostructures: High catalytic selectivity for semihydrogenation of alkynes. J. Am. Chem. Soc. 2017, 139, 7294–7301.
Shao, L. D.; Zhang, W.; Armbrüster, M.; Teschner, D.; Girgsdies, F.; Zhang, B. S.; Timpe, O.; Friedrich, M.; Schlögl, R.; Su, D. S. Nanosizing intermetallic compounds onto carbon nanotubes: Active and selective hydrogenation catalysts. Angew. Chem., Int. Ed. 2011, 50, 10231–10235.
Kyriakou, G.; Boucher, M. B.; Jewell, A. D.; Lewis, E. A.; Lawton, T. J.; Baber, A. E.; Tierney, H. L.; Flytzani-Stephanopoulos, M.; Sykes, E. C. H. Isolated metal atom geometries as a strategy for selective heterogeneous hydrogenations. Science 2012, 335, 1209–1212.
Pei, G. X.; Liu, X. Y.; Wang, A. Q.; Lee, A. F.; Isaacs, M. A.; Li, L.; Pan, X. L.; Yang, X. F.; Wang, X. D.; Tai, Z. J. et al. Ag alloyed Pd single-atom catalysts for efficient selective hydrogenation of acetylene to ethylene in excess ethylene. ACS Catal. 2015, 5, 3717–3725.
Pei, G. X.; Liu, X. Y.; Wang, A. Q.; Li, L.; Huang, Y. Q.; Zhang, T.; Lee, J. W.; Jang, B. W. L.; Mou, C. Y. Promotional effect of Pd single atoms on Au nanoparticles supported on silica for the selective hydrogenation of acetylene in excess ethylene. New J. Chem. 2014, 38, 2043–2051.
Pei, G. X.; Liu, X. Y.; Yang, X. F.; Zhang, L. L.; Wang, A. Q.; Li, L.; Wang, H.; Wang, X. D.; Zhang, T. Performance of Cu-alloyed Pd single-atom catalyst for semihydrogenation of acetylene under simulated front-end conditions. ACS Catal. 2017, 7, 1491–1500.
Kovnir, K.; Armbrüster, M.; Teschner, D.; Venkov, T. V.; Szentmiklósi, L.; Jentoft, F. C.; Knop-Gericke, A.; Grin, Y.; Schlögl, R. In situ surface characterization of the intermetallic compound PdGa—A highly selective hydrogenation catalyst. Surf. Sci. 2009, 603, 1784–1792.
Li, X. T.; Chen, L.; Shang, C.; Liu, Z. P. In situ surface structures of PdAg catalyst and their influence on acetylene semihydrogenation revealed by machine learning and experiment. J. Am. Chem. Soc. 2021, 143, 6281–6292.
Zheng, W. J.; Wang, Y.; Wang, B. J.; Fan, M. H.; Ling, L. X.; Zhang, R. G. C2H2 selective hydrogenation to C2H4: Engineering the surface structure of Pd-based alloy catalysts to adjust the catalytic performance. J. Phys. Chem. C 2021, 125, 15251–15261.
Kim, S. K.; Lee, J. H.; Ahn, I. Y.; Kim, W. J.; Moon, S. H. Performance of Cu-promoted Pd catalysts prepared by adding Cu using a surface redox method in acetylene hydrogenation. App. Catal. A Gen. 2011, 401, 12–19.
Lang, R.; Du, X. R.; Huang, Y. K.; Jiang, X. Z.; Zhang, Q.; Guo, Y. L.; Liu, K. P.; Qiao, B. T.; Wang, A. Q.; Zhang, T. Single-atom catalysts based on the metal-oxide interaction. Chem. Rev. 2020, 120, 11986–12043.
Qiao, B. T.; Wang, A. Q.; Yang, X. F.; Allard, L. F.; Jiang, Z.; Cui, Y. T.; Liu, J. Y.; Li, J.; Zhang, T. Single-atom catalysis of CO oxidation using Pt1/FeOx. Nat. Chem. 2011, 3, 634–641.
Wang, X.; Zhang, Y. W.; Wu, J.; Zhang, Z.; Liao, Q. L.; Kang, Z.; Zhang, Y. Single-atom engineering to ignite 2D transition metal dichalcogenide based catalysis: Fundamentals, progress, and beyond. Chem. Rev. 2022, 122, 1273–1348.
Wei, Y. S.; Zhang, M.; Zou, R. Q.; Xu, Q. Metal-organic framework-based catalysts with single metal sites. Chem. Rev. 2020, 120, 12089–12174.
Wang, Y. X.; Su, H. Y.; He, Y. H.; Li, L. G.; Zhu, S. Q.; Shen, H.; Xie, P. F.; Fu, X. B.; Zhou, G. Y.; Feng, C. et al. Advanced electrocatalysts with single-metal-atom active sites. Chem. Rev. 2020, 120, 12217–12314.
Li, Z. J.; Wang, D. H.; Wu, Y. E.; Li, Y. D. Recent advances in the precise control of isolated single-site catalysts by chemical methods. Natl. Sci. Rev. 2018, 5, 673–689.
Zhou, X. M. TiO2-supported single-atom catalysts for photocatalytic reactions. Acta Phys. Chim. Sin. 2021, 37, 2008064.
Zhang, L. L.; Zhou, M. X.; Wang, A. Q.; Zhang, T. Selective hydrogenation over supported metal catalysts: From nanoparticles to single atoms. Chem. Rev. 2020, 120, 683–733.
Liu, Y. W.; Wang, B. X.; Fu, Q.; Liu, W.; Wang, Y.; Gu, L.; Wang, D. S.; Li, Y. D. Polyoxometalate-based metal-organic framework as molecular sieve for highly selective semi-hydrogenation of acetylene on isolated single Pd atom sites. Angew. Chem., Int. Ed. 2021, 60, 22522–22528.
Liu, P. X.; Zhao, Y.; Qin, R. X.; Mo, S. G.; Chen, G. X.; Gu, L.; Chevrier, D. M.; Zhang, P.; Guo, Q.; Zang, D. D. et al. Photochemical route for synthesizing atomically dispersed palladium catalysts. Science 2016, 352, 797–800.
Wei, S. J.; Li, A.; Liu, J. C.; Li, Z.; Chen, W. X.; Gong, Y.; Zhang, Q. H.; Cheong, W. C.; Wang, Y.; Zheng, L. R. et al. Direct observation of noble metal nanoparticles transforming to thermally stable single atoms. Nat. Nanotechnol. 2018, 13, 856–861.
Ge, X. X.; Zhou, P.; Zhang, Q. H.; Xia, Z. H.; Chen, S. L.; Gao, P.; Zhang, Z.; Gu, L.; Guo, S. J. Palladium single atoms on TiO2 as a photocatalytic sensing platform for analyzing the organophosphorus pesticide chlorpyrifos. Angew. Chem., Int. Ed. 2020, 59, 232–236.
Xu, H. D.; Zhang, Z. H.; Liu, J. X.; Do-Thanh, C. L.; Chen, H.; Xu, S. H.; Lin, Q. J.; Jiao, Y.; Wang, J. L.; Wang, Y. et al. Entropy-stabilized single-atom Pd catalysts via high-entropy fluorite oxide supports. Nat. Commun. 2020, 11, 3908.
Tauster, S. J.; Fung, S. C. Strong metal-support interactions: Occurrence among the binary oxides of groups IIA–VB. J. Catal. 1978, 55, 29–35.
Tauster, S. J.; Fung, S. C.; Garten, R. L. Strong metal-support interactions. Group 8 noble metals supported on titanium dioxide. J. Am. Chem. Soc. 1978, 100, 170–175.
Tauster, S. J. Strong metal-support interactions. Acc. Chem. Res. 1987, 20, 389–394.
Bernal, S.; Calvino, J. J.; Cauqui, M. A.; Gatica, J. M.; Cartes, C. L.; Omil, J. A. P.; Pintado, J. M. Some contributions of electron microscopy to the characterisation of the strong metal-support interaction effect. Catal. Today 2003, 77, 385–406.
Naito, S.; Aida, S.; Kasahara, T.; Miyao, T. Infrared spectroscopic study on the reaction mechanism of CO hydrogenation over Pd/CeO2. Res. Chem. Intermed. 2006, 32, 279–290.
d’Alnoncourt, R. N.; Friedrich, M.; Kunkes, E.; Rosenthal, D.; Girgsdies, F.; Zhang, B. S.; Shao, L. D.; Schuster, M.; Behrens, M.; Schlögl, R. Strong metal-support interactions between palladium and iron oxide and their effect on CO oxidation. J. Catal. 2014, 317, 220–228.
Bernal, S.; Botana, F. J.; Calvino, J. J.; Cifredo, G. A.; Pérez-Omil, J. A.; Pintado, J. M. HREM study of the behaviour of a Rh/CeO2 catalyst under high temperature reducing and oxidizing conditions. Catal. Today 1995, 23, 219–250.
Kast, P.; Friedrich, M.; Teschner, D.; Girgsdies, F.; Lunkenbein, T.; d’Alnoncourt, R. N.; Behrens, M.; Schlögl, R. CO oxidation as a test reaction for strong metal-support interaction in nanostructured Pd/FeO powder catalysts. Appl. Catal. A Gen. 2015, 502, 8–17.
Tauster, S. J.; Fung, S. C.; Baker, R. T. K.; Horsley, J. A. Strong interactions in supported-metal catalysts. Science 1981, 211, 1121–1125.
Liu, X. Y.; Liu, M. H.; Luo, Y. C.; Mou, C. Y.; Lin, S. D.; Cheng, H. K.; Chen, J. M.; Lee, J. F.; Lin, T. S. Strong metal-support interactions between gold nanoparticles and ZnO nanorods in CO oxidation. J. Am. Chem. Soc. 2012, 134, 10251–10258.
Qiao, B. T.; Liang, J. X.; Wang, A. Q.; Xu, C. Q.; Li, J.; Zhang, T.; Liu, J. J. Ultrastable single-atom gold catalysts with strong covalent metal-support interaction (CMSI). Nano Res. 2015, 8, 2913–2924.
Hu, P. P.; Huang, Z. W.; Amghouz, Z.; Makkee, M.; Xu, F.; Kapteijn, F.; Dikhtiarenko, A.; Chen, Y. X.; Gu, X.; Tang, X. F. Electronic metal-support interactions in single-atom catalysts. Angew. Chem., Int. Ed. 2014, 53, 3418–3421.
Bruix, A.; Rodriguez, J. A.; Ramírez, P. J.; Senanayake, S. D.; Evans, J.; Park, J. B.; Stacchiola, D.; Liu, P.; Hrbek, J.; Illas, F. A new type of strong metal-support interaction and the production of H2 through the transformation of water on Pt/CeO2(111) and Pt/CeOx/TiO2(110) catalysts. J. Am. Chem. Soc. 2012, 144, 8968–8974.
Du, X. R.; Huang, Y. K.; Pan, X. L.; Han, B.; Su, Y.; Jiang, Q. K.; Li, M. R.; Tang, H. L.; Li, G.; Qiao, B. T. Size-dependent strong metal-support interaction in TiO2 supported Au nanocatalysts. Nat. Commun. 2020, 11, 5811.
Ma, D. Size-dependent strong metal-support interaction. Acta Phys. Chim. Sin. 2022, 38, 2101039.
Wang, H.; Wang, L.; Lin, D.; Feng, X.; Niu, Y. M.; Zhang, B. S.; Xiao, F. S. Strong metal-support interactions on gold nanoparticle catalysts achieved through Le chatelier’s principle. Nat. Catal. 2021, 4, 418–424.
Zhang, Y. S.; Liu, J. X.; Qian, K.; Jia, A. P.; Li, D.; Shi, L.; Hu, J.; Zhu, J. F.; Huang, W. X. Structure sensitivity of Au-TiO2 strong metal-support interactions. Angew. Chem., Int. Ed. 2021, 60, 12074–12081.
Tang, H. L.; Liu, F.; Wei, J. K.; Qiao, B. T.; Zhao, K. F.; Su, Y.; Jin, C. Z.; Li, L.; Liu, J. Y.; Wang, J. H. et al. Ultrastable hydroxyapatite/titanium-dioxide-supported gold nanocatalyst with strong metal-support interaction for carbon monoxide oxidation. Angew. Chem., Int. Ed. 2016, 55, 10606–10611.
Yang, J. R.; Li, W. H.; Tan, S. D.; Xu, K. N.; Wang, Y.; Wang, D. S.; Li, Y. D. The electronic metal-support interaction directing the design of single atomic site catalysts: Achieving high efficiency towards hydrogen evolution. Angew. Chem., Int. Ed. 2012, 60, 19085–19091.
Dong, J. H.; Fu, Q.; Li, H. B.; Xiao, J. P.; Yang, B.; Zhang, B. S.; Bai, Y. X.; Song, T. Y.; Zhang, R. K.; Gao, L. J. et al. Reaction-induced strong metal-support interactions between metals and inert boron nitride nanosheets. J. Am. Chem. Soc. 2020, 142, 17167–17174.
Li, W. H.; Yang, J. R.; Jing, H. Y.; Zhang, J.; Wang, Y.; Li, J.; Zhao, J.; Wang, D. S.; Li, Y. D. Creating high regioselectivity by electronic metal-support interaction of a single-atomic-site catalyst. J. Am. Chem. Soc. 2021, 143, 15453–15461.
Han, B.; Guo, Y. L.; Huang, Y. K.; Xi, W.; Xu, J.; Luo, J.; Qi, H. F.; Ren, Y. J.; Liu, X. Y.; Qiao, B. T. et al. Strong metal-support interactions between Pt single atoms and TiO2. Angew. Chem., Int. Ed. 2020, 59, 11824–11829.
Chen, F.; Li, T. B.; Pan, X. L.; Guo, Y. L.; Han, B.; Liu, F.; Qiao, B. T.; Wang, A. Q.; Zhang, T. Pd1/CeO2 single-atom catalyst for alkoxycarbonylation of aryl iodides. Sci. China Mater. 2020, 63, 959–964.
Spezzati, G.; Su, Y. Q.; Hofmann, J. P.; Benavidez, A. D.; DeLaRiva, A. T.; McCabe, J.; Datye, A. K.; Hensen, E. J. M. Atomically dispersed Pd-O species on CeO2(111) as highly active sites for low-temperature CO oxidation. ACS Catal. 2017, 7, 6887–6891.
Lou, Y.; Jiang, F.; Zhu, W.; Wang, L.; Yao, T. Y.; Wang, S. S.; Yang, B.; Yang, B.; Zhu, Y. F.; Liu, X. H. CeO2 supported Pd dimers boosting CO2 hydrogenation to ethanol. Appl. Catal. B Environ. 2021, 291, 120122.
Haller, G. L.; Resasco, D. E. Metal-support interaction: Group VIII metals and reducible oxides. Adv. Catal. 1989, 36, 173–235.
Binet, C.; Jadi, A.; Lavalley, J. C.; Boutonnet-Kizling, M. Metal support interaction in Pd/CeO2 catalysts: Fourier-transform infrared studies of the effects of the reduction temperature and metal loading. Part 1.-Catalysts prepared by the microemulsion technique. J. Chem. Soc. Faraday Trans. 1992, 88, 2079–2084.
Trovarelli, A.; Dolcetti, G.; De Leitenburg, C.; Kašpar, J.; Finetti, P.; Santoni, A. Rh−CeO2 Interaction induced by high-temperature reduction. Characterization and catalytic behaviour in transient and continuous conditions. J. Chem. Soc. Faraday Trans. 1992, 88, 1311–1319.
Aitbekova, A.; Wu, L. H.; Wrasman, C. J.; Boubnov, A.; Hoffman, A. S.; Goodman, E. D.; Bare, S. R.; Cargnello, M. Low-temperature restructuring of CeO2-supported Ru nanoparticles determines selectivity in CO2 catalytic reduction. J. Am. Chem. Soc. 2018, 140, 13736–13745.
Jones, J.; Xiong, H. F.; DeLaRiva, A. T.; Peterson, E. J.; Pham, H.; Challa, S. R.; Qi, G.; Oh, S.; Wiebenga, M. H.; Hernández, X. I. P. et al. Thermally stable single-atom platinum-on-ceria catalysts via atom trapping. Science 2016, 353, 150–154.
Newton, M. A.; Belver-Coldeira, C.; Martínez-Arias, A.; Fernández-García, M. “Oxidationless” promotion of rapid palladium redispersion by oxygen during redox CO/(NO+O2) cycling. Angew. Chem., Int. Ed. 2007, 46, 8629–8631.
Jeong, H.; Bae, J.; Han, J. W.; Lee, H. Promoting effects of hydrothermal treatment on the activity and durability of Pd/CeO2 catalysts for CO oxidation. ACS Catal. 2017, 7, 7097–7105.
Xin, P. Y.; Li, J.; Xiong, Y.; Wu, X.; Dong, J. C.; Chen, W. X.; Wang, Y.; Gu, L.; Luo, J.; Rong, H. P. et al. Revealing the active species for aerobic alcohol oxidation by using uniform supported palladium catalysts. Angew. Chem., Int. Ed. 2018, 57, 4642–4646.
Ma, J.; Lou, Y.; Cai, Y. F.; Zhao, Z. Y.; Wang, L.; Zhan, W. C.; Guo, Y. L.; Guo, Y. The relationship between the chemical state of Pd species and the catalytic activity for methane combustion on Pd/CeO2. Catal. Sci. Technol. 2018, 8, 2567–2577.
Chen, Y. X.; Chen, J. X.; Qu, W. Y.; George, C.; Aouine, M.; Vernoux, P.; Tang, X. F. Well-defined palladium-ceria interfacial electronic effects trigger CO oxidation. Chem. Commun. 2018, 54, 10140–10143.
Boronin, A. I.; Slavinskaya, E. M.; Danilova, I. G.; Gulyaev, R. V.; Amosov, Y. I.; Kuznetsov, P. A.; Polukhina, I. A.; Koscheev, S. V.; Zaikovskii, V. I.; Noskov, A. S. Investigation of palladium interaction with cerium oxide and its state in catalysts for low-temperature CO oxidation. Catal. Today 2009, 144, 201–211.
Gulyaev, R. V.; Stadnichenko, A. I.; Slavinskaya, E. M.; Ivanova, A. S.; Koscheev, S. V.; Boronin, A. I. In situ preparation and investigation of Pd/CeO2 catalysts for the low-temperature oxidation of CO. Appl. Catal. A Gen. 2012, 439–440, 41–50.
Zhang, Y. H.; Cai, Y. F.; Guo, Y.; Wang, H. F.; Wang, L.; Lou, Y.; Guo, Y. L.; Lu, G. Z.; Wang, Y. Q. The effects of the Pd chemical state on the activity of Pd/Al2O3 catalysts in CO oxidation. Catal. Sci. Technol. 2014, 4, 3973–3980.
Luo, Y.; Villaseca, S. A.; Friedrich, M.; Teschner, D.; Knop-Gericke, A.; Armbrüster, M. Addressing electronic effects in the semi-hydrogenation of ethyne by in Pd2 and intermetallic Ga-Pd compounds. J. Catal. 2016, 338, 265–272.
Huang, F.; Deng, Y. C.; Chen, Y. L.; Cai, X. B.; Peng, M.; Jia, Z. M.; Ren, P. J.; Xiao, D. Q.; Wen, X. D.; Wang, N. et al. Atomically dispersed Pd on nanodiamond/graphene hybrid for selective hydrogenation of acetylene. J. Am. Chem. Soc. 2018, 140, 13142–13146.
Armbrüster, M.; Kovnir, K.; Behrens, M.; Teschner, D.; Grin, Y.; Schlögl, R. Pd-Ga intermetallic compounds as highly selective semihydrogenation catalysts. J. Am. Chem. Soc. 2010, 132, 14745–14747.
Armbrüster, M.; Wowsnick, G.; Friedrich, M.; Heggen, M.; Cardoso-Gil, R. Synthesis and catalytic properties of nanoparticulate intermetallic Ga-Pd compounds. J. Am. Chem. Soc. 2011, 133, 9112–9118.
Vilé, G.; Bridier, B.; Wichert, J.; Pérez-Ramírez, J. Ceria in hydrogenation catalysis: High selectivity in the conversion of alkynes to olefins. Angew. Chem., Int. Ed. 2012, 51, 8620–8623.
Haller, G. L. New catalytic concepts from new materials: Understanding catalysis from a fundamental perspective, past, present, and future. J. Catal. 2003, 216, 12–22.
Vignola, E.; Steinmann, S. N.; Al Farra, A.; Vandegehuchte, B. D.; Curulla, D.; Sautet, P. Evaluating the risk of C−C bond formation during selective hydrogenation of acetylene on palladium. ACS Catal. 2018, 8, 1662–1671.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (Nos. 21972135, 21961142006, and 51701201), CAS Project for Young Scientists in Basic Research (No. YSBR-022), and the National Key Research and Development Program of China (No. 2021YFA1500503).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Guo, Y., Li, Y., Du, X. et al. Pd single-atom catalysts derived from strong metal-support interaction for selective hydrogenation of acetylene. Nano Res. 15, 10037–10043 (2022). https://doi.org/10.1007/s12274-022-4376-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-022-4376-5