Abstract
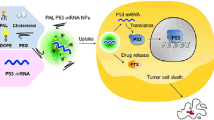
Convincing evidence indicates that the existence of cancer stem cells (CSCs) within malignant tumors is mostly responsible for the failure of chemotherapy. Therefore, instead of merely targeting bulk cancer cells, simultaneous elimination of both CSCs and bulk cancer cells is necessary to improve therapeutic outcomes. Herein, we designed cationic-lipid-assisted nanoparticles DTXLNPsiRNA for simultaneous encapsulation of the conventional chemotherapeutic agentdocetaxel (DTXL) and small interfering RNA (siRNA) targeting BMI-1 (siBMI-1). We confirmed that nanoparticles DTXLNPsiBMI-1 effectively deliver both therapeutic agents into CSCs and bulk cancer cells. The bulk cancer cells were effectively killed by the DTXL encapsulated in DTXLNPsiBMI-1. In breast CSCs, BMI-1 expression was significantly downregulated by DTXLNPsiBMI-1; consequently, the stemness was reduced and chemosensitivity of CSCs to DTXL was enhanced, resulting in the elimination of CSCs. Therefore, via DTXLNPsiBMI-1, the combination of siBMI-1 and DTXL completely inhibited tumor growth and prevented a relapse by synergistic killing of CSCs and bulk cancer cells in a murine model of an MDA-MB-231 orthotropic tumor.

Similar content being viewed by others
References
Clevers, H. The cancer stem cell: Premises, promises and challenges. Nat. Med. 2011, 17, 313–319.
Reya, T.; Morrison, S. J.; Clarke, M. F.; Weissman, I. L. Stem cells, cancer, and cancer stem cells. Nature 2001, 414, 105–111.
Creighton, C. J.; Li, X. X.; Landis, M.; Dixon, J. M.; Neumeister, V. M.; Sjolund, A.; Rimm, D. L.; Wong, H.; Rodriguez, A.; Herschkowitz, J. I. et al. Residual breast cancers after conventional therapy display mesenchymal as well as tumor-initiating features. Proc. Natl. Acad. Sci. USA 2009, 106, 13820–13825.
Gottesman, M. M.; Fojo, T.; Bates, S. E. Multidrug resistance in cancer: Role of ATP-dependent transporters. Nat. Rev. Cancer 2002, 2, 48–58.
Dean, M.; Fojo, T.; Bates, S. Tumour stem cells and drug resistance. Nat. Rev. Cancer 2005, 5, 275–284.
Venezia, T. A.; Merchant, A. A.; Ramos, C. A.; Whitehouse, N. L.; Young, A. S.; Shaw, C. A.; Goodell, M. A. Molecular signatures of proliferation and quiescence in hematopoietic stem cells. PLoS Biol. 2004, 2, e301.
Li, X. X.; Lewis, M. T.; Huang, J.; Gutierrez, C.; Osborne, C. K.; Wu, M. F.; Hilsenbeck, S. G.; Pavlick, A.; Zhang, X. M.; Chamness, G. C. et al. Intrinsic resistance of tumorigenic breast cancer cells to chemotherapy. J. Natl. Cancer Inst. 2008, 100, 672–679.
Li, Y. Z.; Rogoff, H. A.; Keates, S.; Gao, Y.; Murikipudi, S.; Mikule, K.; Leggett, D.; Li, W.; Pardee, A. B.; Li, C. J. Suppression of cancer relapse and metastasis by inhibiting cancer stemness. Proc. Natl. Acad. Sci. USA 2015, 112, 1839–1844.
Li, R. J.; Ying, X.; Zhang, Y.; Ju, R. J.; Wang, X. X.; Yao, H. J.; Men, Y.; Tian, W.; Yu, Y.; Zhang, L. et al. All-trans retinoic acid stealth liposomes prevent the relapse of breast cancer arising from the cancer stem cells. J. Control Release 2011, 149, 281–291.
Song, Y. J.; Zhang, S. S.; Guo, X. L.; Sun, K.; Han, Z. P.; Li, R.; Zhao, Q. D.; Deng, W. J.; Xie, X. Q.; Zhang, J. W. et al. Autophagy contributes to the survival of CD133+ liver cancer stem cells in the hypoxic and nutrient-deprived tumor microenvironment. Cancer Lett. 2013, 339, 70–81.
Viale, A.; De Franco, F.; Orleth, A.; Cambiaghi, V.; Giuliani, V.; Bossi, D.; Ronchini, C.; Ronzoni, S.; Muradore, I.; Monestiroli, S. et al. Cell-cycle restriction limits DNA damage and maintains self-renewal of leukaemia stem cells. Nature 2009, 457, 51–56.
McAuliffe, S. M., Morgan, S. L.; Wyant, G. A.; Tran, L. T.; Muto, K. W.; Chen, Y. S.; Chin, K. T.; Partridge, J. C.; Poole, B. B.; Cheng, K. H. et al. Targeting notch, a key pathway for ovarian cancer stem cells, sensitizes tumors to platinum therapy. Proc. Natl. Acad. Sci. USA 2012, 109, E2939–E2948.
van der Lugt, N. M.; Domen, J.; Linders, K.; van Roon, M.; Robanus-Maandag, E.; te Riele, H.; van der Valk, M.; Deschamps, J.; Sofroniew, M.; van Lohuizen, M. Posterior transformation, neurological abnormalities, and severe hematopoietic defects in mice with a targeted deletion of the bmi-1 proto-oncogene. Genes Dev. 1994, 8, 757–769.
Yu, T. X.; Chen, X.; Zhang, W.; Colon, D.; Shi, J. D.; Napier, D.; Rychahou, P.; Lu, W. G.; Lee, E. Y.; Weiss, H. L. et al. Regulation of the potential marker for intestinal cells, Bmi1, by β-catenin and the zinc finger protein KLF4: Implications for colon cancer. J. Biol. Chem. 2012, 287, 3760–3768.
Lessard, J.; Sauvageau, G. Bmi-1 determines the proliferative capacity of normal and leukaemic stem cells. Nature 2003, 423, 255–260.
Szotek, P. P.; Pieretti-Vanmarcke, R.; Masiakos, P. T.; Dinulescu, D. M.; Connolly, D.; Foster, R.; Dombkowski, D.; Preffer, F.; MacLaughlin, D. T.; Donahoe, P. K. Ovarian cancer side population defines cells with stem cell-like characteristics and Mullerian Inhibiting Substance responsiveness. Proc. Natl. Acad. Sci. USA 2006, 103, 11154–11159.
Lukacs, R. U.; Memarzadeh, S.; Wu, H.; Witte, O. N. BMI-1 is a crucial regulator of prostate stem cell self-renewal and malignant transformation. Cell Stem Cell 2010, 7, 682–693.
Chiba, T.; Miyagi, S.; Saraya, A.; Aoki, R.; Seki, A.; Morita, Y.; Yonemitsu, Y.; Yokosuka, O.; Taniguchi, H.; Nakauchi, H. et al. The polycomb gene product BMI1 contributes to the maintenance of tumor-initiating side population cells in hepatocellular carcinoma. Cancer Res. 2008, 68, 7742–7749.
Jin, M.; Zhang, T.; Liu, C.; Badeaux, M. A.; Liu, B. G.; Liu, R. F.; Jeter, C.; Chen, X.; Vlassov, A. V.; Tang, D. G. miRNA-128 suppresses prostate cancer by inhibiting BMI-1 to inhibit tumor-initiating cells. Cancer Res. 2014, 74, 4183–4195.
Liu, J.; Cao, L.; Chen, J. C.; Song, S. W.; Lee, I. H.; Quijano, C.; Liu, H. J.; Keyvanfar, K.; Chen, H. Q.; Cao, L. Y. et al. Bmi1 regulates mitochondrial function and the DNA damage response pathway. Nature 2009, 459, 387–392.
Wei, X. L.; Dou, X. W.; Bai, J. W.; Luo, X. R.; Qiu, S. Q.; Xi, D. D.; Huang, W. H.; Du, C. W.; Man, K.; Zhang, G. J. ERα inhibits epithelial-mesenchymal transition by suppressing Bmi1 in breast cancer. Oncotarget 2015, 6, 21704–21717.
Chen, D. M.; Wu, M. S.; Li, Y.; Chang, I.; Yuan, Q.; Ekimyan-Salvo, M.; Deng, P.; Yu, B.; Yu, Y. X.; Dong, J. Q. et al. Targeting BMI1+ cancer stem cells overcomes chemoresistance and inhibits metastases in squamous cell carcinoma. Cell Stem Cell 2017, 20, 621–634.e6.
Kreso, A.; van Galen, P.; Pedley, N. M.; Lima-Fernandes, E.; Frelin, C.; Davis, T.; Cao, L. X.; Baiazitov, R.; Du, W.; Sydorenko, N. et al. Self-renewal as a therapeutic target in human colorectal cancer. Nat. Med. 2014, 20, 29–36.
Jackson, A. L.; Linsley, P. S. Recognizing and avoiding siRNA off-target effects for target identification and therapeutic application. Nat. Rev. Drug Discov. 2010, 9, 57–67.
Davis, M. E.; Zuckerman, J. E.; Choi, C. H. J.; Seligson, D.; Tolcher, A.; Alabi, C. A.; Yen, Y.; Heidel, J. D.; Ribas, A. Evidence of RNAi in humans from systemically administered siRNA via targeted nanoparticles. Nature 2010, 464, 1067–1070.
Fire, A.; Xu, S. Q.; Montgomery, M. K.; Kostas, S. A.; Driver, S. E.; Mello, C. C. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 1998, 391, 806–811.
Zamore, P. D.; Tuschl, T.; Sharp, P. A.; Bartel, D. P. RNAi: Double-stranded RNA directs the ATP-dependent cleavage of mRNA at 21 to 23 nucleotide intervals. Cell 2000, 101, 25–33.
Hu, T. S.; Liu, S. R.; Breiter, D. R.; Wang, F.; Tang, Y.; Sun, S. H. Octamer 4 small interfering RNA results in cancer stem cell-like cell apoptosis. Cancer Res. 2008, 68, 6533–6540.
Dominska, M.; Dykxhoorn, D. M. Breaking down the barriers: siRNA delivery and endosome escape. J. Cell Sci. 2010, 123, 1183–1189.
Layzer, J. M.; McCaffrey, A. P.; Tanner, A. K.; Huang, Z.; Kay, M. A.; Sullenger B. A. In vivo activity of nuclease-resistant siRNAs. RNA 2004, 10, 766–771.
Judge, A. D.; Sood, V.; Shaw, J. R.; Fang, D.; McClintock, K.; MacLachlan, I. Sequence-dependent stimulation of the mammalian innate immune response by synthetic siRNA. Nat. Biotechnol. 2005, 23, 457–462.
Chang, J. C.; Wooten, E. C.; Tsimelzon, A.; Hilsenbeck, S. G.; Gutierrez, M. C.; Tham, Y. L.; Kalidas, M.; Elledge, R.; Mohsin, S.; Osborne, C. K. et al. Patterns of resistance and incomplete response to docetaxel by gene expression profiling in breast cancer patients. J. Clin. Oncol. 2005, 23, 1169–1177.
Galletti, E.; Magnani, M.; Renzulli, M. L.; Botta, M. Paclitaxel and docetaxel resistance: Molecular mechanisms and development of new generation taxanes. ChemMedChem 2007, 2, 920–942.
Wang, H.-X.; Zuo, Z.-Q.; Du, J.-Z.; Wang, Y.-C.; Sun, R.; Cao, Z.-T.; Ye, X.-D.; Wang, J.-L.; Leong, K. W.; Wang, J. Surface charge critically affects tumor penetration and therapeutic efficacy of cancer nanomedicines. Nano Today 2016, 11, 133–144.
Gao, Z. B.; Zhang, L.; Sun, Y. J. Nanotechnology applied to overcome tumor drug resistance. J. Control Release 2012, 162, 45–55.
Torchilin, V. Tumor delivery of macromolecular drugs based on the EPR effect. Adv. Drug Deliver. Rev. 2011, 63, 131–135.
Hobbs, S. K.; Monsky, W. L.; Yuan, F.; Roberts, W. G.; Griffith, L.; Torchilin, V. P.; Jain, R. K. Regulation of transport pathways in tumor vessels: Role of tumor type and microenvironment. Proc. Natl. Acad. Sci. USA 1998, 95, 4607–4612.
Yang, Z.; Gao, D.; Cao, Z.; Cheng, D.; Liu, J.; Shuai, X. T. Drug and gene co-delivery systems for cancer treatment. Biomater. Sci. 2015, 3, 1035–1049.
Song, X. J.; Feng, L. Z.; Liang, C.; Gao, M.; Song, G. S.; Liu, Z. Liposomes co-loaded with metformin and chlorin e6 modulate tumor hypoxia during enhanced photodynamic therapy. Nano Res. 2017, 10, 1200–1212.
Yang, C. B.; Chan, K. K.; Lin, W. J.; Soehartono, A. M.; Lin, G. M.; Toh, H.; Yoon, H. S.; Chen, C. K.; Yong K. T. Biodegradable nanocarriers for small interfering ribonucleic acid (siRNA) co-delivery strategy increase the chemosensitivity of pancreatic cancer cells to gemcitabine. Nano Res. 2017, 10, 3049–3067.
Kutty, R. V., Tay, C. Y., Lim, C. S.; Feng S. S.; Leong, D. T. Anti-migratory and increased cytotoxic effects of novel dual drug-loaded complex hybrid micelles in triple negative breast cancer cells. Nano Res. 2015, 8, 2533–2547.
Xu, C. N.; Tian, H. Y.; Wang, P.; Wang, Y. B.; Chen, X. S. The suppression of metastatic lung cancer by pulmonary admini-stration of polymer nanoparticles for co-delivery of doxorubicin and survivin siRNA. Biomater. Sci. 2016, 4, 1646–1654.
Yang, X. Z.; Dou, S.; Sun, T. M.; Mao, C. Q.; Wang, H. X.; Wang, J. Systemic delivery of siRNA with cationic lipid assisted PEG-PLA nanoparticles for cancer therapy. J. Control Release 2011, 156, 203–211.
Zuo, Z. Q.; Chen, K. G.; Yu, X. Y.; Zhao, G.; Shen, S.; Cao, Z. T.; Luo, Y. L.; Wang, Y. C.; Wang, J. Promoting tumor penetration of nanoparticles for cancer stem cell therapy by TGF-β signaling pathway inhibition. Biomaterials 2016, 82, 48–59.
Xu, C.-F.; Liu, Y.; Shen, S.; Zhu, Y.-H.; Wang, J. Targeting glucose uptake with siRNA-based nanomedicine for cancer therapy. Biomaterials 2015, 51, 1–11.
Sun, R.; Shen, S.; Zhang, Y.-J.; Xu, C.-F.; Cao, Z.-T.; Wen, L.-P.; Wang, J. Nanoparticle-facilitated autophagy inhibition promotes the efficacy of chemotherapeutics against breast cancer stem cells. Biomaterials 2016, 103, 44–55.
Yu, F. Y.; Yao, H. R.; Zhu, P. C.; Zhang, X. Q.; Pan, Q. H.; Gong, C.; Huang, Y. J.; Hu, X. Q.; Su, F. X.; Lieberman, J. et al. let-7 regulates self renewal and tumorigenicity of breast cancer cells. Cell 2007, 131, 1109–1123.
Chou, T. C. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res. 2010, 70, 440–446.
Ginestier, C.; Hur, M. H.; Charafe-Jauffret, E.; Monville, F.; Dutcher, J.; Brown, M.; Jacquemier, J.; Viens, P.; Kleer, C. G.; Liu, S. L. et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell 2007, 1, 555–567.
Douville, J.; Beaulieu, R.; Balicki, D. ALDH1 as a functional marker of cancer stem and progenitor cells. Stem Cells Dev. 2009, 18, 17–26.
Ma, F.; Li, H. H.; Li, Y. Q.; Ding, X. Y.; Wang, H. J.; Fan, Y.; Lin, C.; Qian, H. L.; Xu, B. H. Aldehyde dehydrogenase 1 (ALDH1) expression is an independent prognostic factor in triple negative breast cancer (TNBC). Medicine 2017, 96, e6561.
Acknowledgements
This work was partially supported by the National Key R&D Program of China (No. 2017YFA0205600), the National Basic Research Program of China (No. 2015CB932100), the National Natural Science Foundation of China (Nos. 51390482, 51633008, and 31470965), National Postdoctoral Program for Innovative Talents (No. BX201700080) and China Postdoctoral Science Foundation (No. 2017M622676).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
12274_2018_2007_MOESM1_ESM.pdf
Simultaneous elimination of cancer stem cells and bulk cancer cells by cationic-lipid-assisted nanoparticles for cancer therapy
Rights and permissions
About this article
Cite this article
Chen, K., Shen, S., Zhao, G. et al. Simultaneous elimination of cancer stem cells and bulk cancer cells by cationic-lipid-assisted nanoparticles for cancer therapy. Nano Res. 11, 4183–4198 (2018). https://doi.org/10.1007/s12274-018-2007-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-018-2007-y