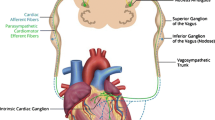
Abstract
Low-level transcutaneous vagus nerve stimulation at the tragus (LLTS) is anti-adrenergic. We aimed to evaluate the acute effects of LLTS on left ventricular (LV) function and autonomic tone. Patients with diastolic dysfunction and preserved LV ejection fraction were enrolled in a prospective, randomized, double-blind, 2 × 2 cross-over study. Patients received two separate, 1-h sessions, at least 1 day apart, of active LLTS (20 Hz, 1 mA below the discomfort threshold) and sham stimulation. Echocardiography was performed after LLTS or sham stimulation to assess cardiac function. A 5-min ECG was performed to assess heart rate variability (HRV). Twenty-four patients were enrolled. LV global longitudinal strain improved by 1.8 ± 0.9% during active LLTS compared to sham stimulation (p = 0.001). Relative to baseline, HRV frequency domain components (low frequency, high frequency, and their ratio) were favorably altered after LLTS compared to sham stimulation (all p < 0.05). We concluded that LLTS acutely ameliorates cardiac mechanics by modulating the autonomic tone. Trial registration: NCT02983448




Similar content being viewed by others
Abbreviations
- HFpEF:
-
Heart failure with preserved ejection fraction
- LV:
-
Left ventricle
- HFrEF:
-
Heart failure with reduced ejection fraction
- VNS:
-
Vagus nerve stimulation
- LLTS:
-
Low-level transcutaneous vagus nerve stimulation
- TENS:
-
Transcutaneous electrical nerve stimulation
- GLS:
-
Global longitudinal strain
- HRV:
-
Heart rate variability
- HF:
-
High frequency
- LF:
-
Low frequency
References
Lam, C. S., Donal, E., Kraigher-Krainer, E., & Vasan, R. S. (2011). Epidemiology and clinical course of heart failure with preserved ejection fraction. European Journal of Heart Failure, 13(1), 18–28.
Owan, T. E., Hodge, D. O., Herges, R. M., Jacobsen, S. J., Roger, V. L., & Redfield, M. M. (2006). Trends in prevalence and outcome of heart failure with preserved ejection fraction. The New England Journal of Medicine, 355(3), 251–259.
Fonarow, G. C., Stough, W. G., Abraham, W. T., Albert, N. M., Gheorghiade, M., Greenberg, B. H., O'Connor, C. M., Sun, J. L., Yancy, C. W., & Young, J. B. (2007). Characteristics, treatments, and outcomes of patients with preserved systolic function hospitalized for heart failure: a report from the OPTIMIZE-HF Registry. Journal of the American College of Cardiology, 50(8), 768–777.
Tribouilloy, C., Rusinaru, D., Mahjoub, H., Souliere, V., Levy, F., Peltier, M., Slama, M., & Massy, Z. (2008). Prognosis of heart failure with preserved ejection fraction: a 5 year prospective population-based study. European Heart Journal, 29(3), 339–347.
Butler, J., Fonarow, G. C., Zile, M. R., Lam, C. S., Roessig, L., Schelbert, E. B., Shah, S. J., Ahmed, A., Bonow, R. O., Cleland, J. G., Cody, R. J., Chioncel, O., Collins, S. P., Dunnmon, P., Filippatos, G., Lefkowitz, M. P., Marti, C. N., McMurray, J. J., Misselwitz, F., Nodari, S., O'Connor, C., Pfeffer, M. A., Pieske, B., Pitt, B., Rosano, G., Sabbah, H. N., Senni, M., Solomon, S. D., Stockbridge, N., Teerlink, J. R., Georgiopoulou, V. V., & Gheorghiade, M. (2014). Developing therapies for heart failure with preserved ejection fraction: current state and future directions. JACC Heart Failure, 2(2), 97–112.
Senni, M., Paulus, W. J., Gavazzi, A., Fraser, A. G., Diez, J., Solomon, S. D., Smiseth, O. A., Guazzi, M., Lam, C. S., Maggioni, A. P., Tschope, C., Metra, M., Hummel, S. L., Edelmann, F., Ambrosio, G., Stewart Coats, A. J., Filippatos, G. S., Gheorghiade, M., Anker, S. D., Levy, D., Pfeffer, M. A., Stough, W. G., & Pieske, B. M. (2014). New strategies for heart failure with preserved ejection fraction: the importance of targeted therapies for heart failure phenotypes. European Heart Journal, 35(40), 2797–2815.
Glezeva, N., & Baugh, J. A. (2014). Role of inflammation in the pathogenesis of heart failure with preserved ejection fraction and its potential as a therapeutic target. Heart Failure Reviews, 19(5), 681–694.
Gomberg-Maitland, M., Shah, S. J., & Guazzi, M. (2016). Inflammation in heart failure with preserved ejection fraction: time to put out the fire. JACC Heart Failure, 4(4), 325–328.
Paulus, W. J., & Tschope, C. (2013). A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. Journal of the American College of Cardiology, 62(4), 263–271.
Kraigher-Krainer, E., Shah, A. M., Gupta, D. K., Santos, A., Claggett, B., Pieske, B., Zile, M. R., Voors, A. A., Lefkowitz, M. P., Packer, M., McMurray, J. J., Solomon, S. D., & Investigators, P. (2014). Impaired systolic function by strain imaging in heart failure with preserved ejection fraction. Journal of the American College of Cardiology, 63(5), 447–456.
Aikawa, T., Naya, M., Obara, M., Manabe, O., Tomiyama, Y., Magota, K., Yamada, S., Katoh, C., Tamaki, N., & Tsutsui, H. (2017). Impaired myocardial sympathetic innervation is associated with diastolic dysfunction in heart failure with preserved ejection fraction: (11)C-hydroxyephedrine PET study. Journal of Nuclear Medicine, 58(5), 784–790.
Toledo, C., Andrade, D. C., Lucero, C., Arce-Alvarez, A., Diaz, H. S., Aliaga, V., Schultz, H. D., Marcus, N. J., Manriquez, M., Faundez, M., & Del Rio, R. (2017). Cardiac diastolic and autonomic dysfunction are aggravated by central chemoreflex activation in heart failure with preserved ejection fraction rats. The Journal of Physiology, 595(8), 2479–2495.
Pavlov, V. A., & Tracey, K. J. (2015). Neural circuitry and immunity. Immunologic Research, 63(1–3), 38–57.
Tracey, K. J. (2009). Reflex control of immunity. Nature Reviews. Immunology, 9(6), 418–428.
Beaumont, E., Wright, G. L., Southerland, E. M., Li, Y., Chui, R., KenKnight, B. H., Armour, J. A., & Ardell, J. L. (2016). Vagus nerve stimulation mitigates intrinsic cardiac neuronal remodeling and cardiac hypertrophy induced by chronic pressure overload in guinea pig. American Journal of Physiology. Heart and Circulatory Physiology, 310(10), H1349–H1359.
Fallgatter, A. J., Neuhauser, B., Herrmann, M. J., Ehlis, A. C., Wagener, A., Scheuerpflug, P., Reiners, K., & Riederer, P. (2003). Far field potentials from the brain stem after transcutaneous vagus nerve stimulation. Journal of Neural Transmission, 110(12), 1437–1443.
Stavrakis, S., Humphrey, M. B., Scherlag, B. J., Hu, Y., Jackman, W. M., Nakagawa, H., Lockwood, D., Lazzara, R., & Po, S. S. (2015). Low-level transcutaneous electrical vagus nerve stimulation suppresses atrial fibrillation. Journal of the American College of Cardiology, 65(9), 867–875.
Yu, L., Huang, B., Po, S. S., Tan, T., Wang, M., Zhou, L., Meng, G., Yuan, S., Zhou, X., Li, X., Wang, Z., Wang, S., & Jiang, H. (2017). Low-level Tragus stimulation for the treatment of ischemia and reperfusion injury in patients with ST-segment elevation myocardial infarction: a proof-of-concept study. JACC. Cardiovascular Interventions, 10(15), 1511–1520.
Clancy, J. A., Mary, D. A., Witte, K. K., Greenwood, J. P., Deuchars, S. A., & Deuchars, J. (2014). Non-invasive vagus nerve stimulation in healthy humans reduces sympathetic nerve activity. Brain Stimulation, 7(6), 871–877.
Nagueh, S. F., Smiseth, O. A., Appleton, C. P., Byrd, B. F., 3rd, Dokainish, H., Edvardsen, T., Flachskampf, F. A., Gillebert, T. C., Klein, A. L., Lancellotti, P., Marino, P., Oh, J. K., Alexandru Popescu, B., Waggoner, A. D., Houston, T., Oslo, N., Phoenix, A., Nashville, T., Hamilton, O. C., Uppsala, S., Ghent, Liege, B., Cleveland, O., Novara, I., Rochester, M., Bucharest, R., & St. Louis, M. (2016). Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. European Heart Journal Cardiovascular Imaging, 17(12), 1321–1360.
Peuker, E. T., & Filler, T. J. (2002). The nerve supply of the human auricle. Clinical Anatomy, 15(1), 35–37.
Tarvainen, M. P., Niskanen, J. P., Lipponen, J. A., Ranta-Aho, P. O., & Karjalainen, P. A. (2014). Kubios HRV—heart rate variability analysis software. Computer Methods and Programs in Biomedicine, 113(1), 210–220.
(1996). Heart rate variability: standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology, Circulation, 93(5) 1043–65.
Laborde, S., Mosley, E., & Thayer, J. F. (2017). Heart rate variability and cardiac vagal tone in psychophysiological research—recommendations for experiment planning, data analysis, and data reporting. Frontiers in Psychology, 8, 213.
Freeman, R. (2006). Assessment of cardiovascular autonomic function. Clinical Neurophysiology, 117(4), 716–730.
Mordi, I. R., Singh, S., Rudd, A., Srinivasan, J., Frenneaux, M., Tzemos, N., & Dawson, D. K. (2018). Comprehensive echocardiographic and cardiac magnetic resonance evaluation differentiates among heart failure with preserved ejection fraction patients, hypertensive patients, and healthy control subjects. JACC: Cardiovascular Imaging, 11(4), 577–585.
Deuchars, S. A., Lall, V. K., Clancy, J., Mahadi, M., Murray, A., Peers, L., & Deuchars, J. (2018). Mechanisms underpinning sympathetic nervous activity and its modulation using transcutaneous vagus nerve stimulation. Experimental Physiology, 103(3), 326–331.
Schwartz, P. J., Pagani, M., Lombardi, F., Malliani, A., & Brown, A. M. (1973). A cardiocardiac sympathovagal reflex in the cat. Circulation Research, 32(2), 215–220.
Frangos, E., Ellrich, J., & Komisaruk, B. R. (2015). Non-invasive access to the vagus nerve central projections via electrical stimulation of the external ear: fMRI evidence in humans. Brain Stimulation, 8(3), 624–636.
Grassi, G., Seravalle, G., Quarti-Trevano, F., Dell'Oro, R., Arenare, F., Spaziani, D., & Mancia, G. (2009). Sympathetic and baroreflex cardiovascular control in hypertension-related left ventricular dysfunction. Hypertension, 53(2), 205–209.
Toschi-Dias, E., Rondon, M., Cogliati, C., Paolocci, N., Tobaldini, E., & Montano, N. (2017). Contribution of autonomic reflexes to the hyperadrenergic state in heart failure. Frontiers in Neuroscience, 11, 162.
Vasudevan, N. T., Mohan, M. L., Goswami, S. K., & Naga Prasad, S. V. (2011). Regulation of beta-adrenergic receptor function: an emphasis on receptor resensitization. Cell Cycle, 10(21), 3684–3691.
Ardell, J. L., Rajendran, P. S., Nier, H. A., KenKnight, B. H., & Armour, J. A. (2015). Central-peripheral neural network interactions evoked by vagus nerve stimulation: functional consequences on control of cardiac function. American Journal of Physiology. Heart and Circulatory Physiology, 309(10), H1740–H1752.
Potter, E., & Marwick, T. H. (2018). Assessment of left ventricular function by echocardiography: the case for routinely adding global longitudinal strain to ejection fraction. JACC: Cardiovascular Imaging, 11(2 Pt 1), 260–274.
Saito, M., Khan, F., Stoklosa, T., Iannaccone, A., Negishi, K., & Marwick, T. H. (2016). Prognostic implications of LV strain risk score in asymptomatic patients with hypertensive heart disease. JACC: Cardiovascular Imaging, 9(8), 911–921.
Ho, S. Y. (2009). Anatomy and myoarchitecture of the left ventricular wall in normal and in disease. European Journal of Echocardiography, 10(8), iii3–iii7.
Schroder, J., Hamada, S., Altiok, E., Almalla, M., Koutziampasi, C., Napp, A., Keszei, A., Hein, M., & Becker, M. (2017). Detection of acute changes in left ventricular function by myocardial deformation analysis after excessive alcohol ingestion. Journal of the American Society of Echocardiography, 30(3), 235–243 e1.
Dedobbeleer, C., Hadefi, A., Naeije, R., & Unger, P. (2013). Left ventricular adaptation to acute hypoxia: a speckle-tracking echocardiography study. Journal of the American Society of Echocardiography, 26(7), 736–745.
Sha, Y., Scherlag, B. J., Yu, L., Sheng, X., Jackman, W. M., Lazzara, R., & Po, S. S. (2011). Low-level right vagal stimulation: anticholinergic and antiadrenergic effects. Journal of Cardiovascular Electrophysiology, 22(10), 1147–1153.
Chinda, K., Tsai, W. C., Chan, Y. H., Lin, A. Y., Patel, J., Zhao, Y., Tan, A. Y., Shen, M. J., Lin, H., Shen, C., Chattipakorn, N., Rubart-von der Lohe, M., Chen, L. S., Fishbein, M. C., Lin, S. F., Chen, Z., & Chen, P. S. (2016). Intermittent left cervical vagal nerve stimulation damages the stellate ganglia and reduces the ventricular rate during sustained atrial fibrillation in ambulatory dogs. Heart Rhythm, 13(3), 771–780.
Tadic, M., Cuspidi, C., Pencic, B., Pavlovic, S. U., Ivanovic, B., Kocijancic, V., & Celic, V. (2015). Association between left ventricular mechanics and heart rate variability in untreated hypertensive patients. Journal of Clinical Hypertension (Greenwich, Conn.), 17(2), 118–125.
Tadic, M., Zlatanovic, M., Cuspidi, C., Ivanovic, B., Stevanovic, A., Damjanov, N., Kocijancic, V., & Celic, V. (2017). The relationship between left ventricular deformation and heart rate variability in patients with systemic sclerosis: two- and three-dimensional strain analysis. International Journal of Cardiology, 236, 145–150.
Gold, M. R., Van Veldhuisen, D. J., Hauptman, P. J., Borggrefe, M., Kubo, S. H., Lieberman, R. A., Milasinovic, G., Berman, B. J., Djordjevic, S., Neelagaru, S., Schwartz, P. J., Starling, R. C., & Mann, D. L. (2016). Vagus nerve stimulation for the treatment of heart failure: the INOVATE-HF Trial. Journal of the American College of Cardiology, 68(2), 149–158.
Zannad, F., De Ferrari, G. M., Tuinenburg, A. E., Wright, D., Brugada, J., Butter, C., Klein, H., Stolen, C., Meyer, S., Stein, K. M., Ramuzat, A., Schubert, B., Daum, D., Neuzil, P., Botman, C., Castel, M. A., D'Onofrio, A., Solomon, S. D., Wold, N., & Ruble, S. B. (2015). Chronic vagal stimulation for the treatment of low ejection fraction heart failure: results of the NEural Cardiac TherApy foR Heart Failure (NECTAR-HF) randomized controlled trial. European Heart Journal, 36(7), 425–433.
Premchand, R. K., Sharma, K., Mittal, S., Monteiro, R., Dixit, S., Libbus, I., DiCarlo, L. A., Ardell, J. L., Rector, T. S., Amurthur, B., KenKnight, B. H., & Anand, I. S. (2014). Autonomic regulation therapy via left or right cervical vagus nerve stimulation in patients with chronic heart failure: results of the ANTHEM-HF trial. Journal of Cardiac Failure, 20(11), 808–816.
Ardell, J. L., Nier, H., Hammer, M., Southerland, E. M., Ardell, C. L., Beaumont, E., KenKnight, B. H., & Armour, J. A. (2017). Defining the neural fulcrum for chronic vagus nerve stimulation: implications for integrated cardiac control. The Journal of Physiology, 595(22), 6887–6903.
Salavatian, S., Beaumont, E., Longpre, J. P., Armour, J. A., Vinet, A., Jacquemet, V., Shivkumar, K., & Ardell, J. L. (2016). Vagal stimulation targets select populations of intrinsic cardiac neurons to control neurally induced atrial fibrillation. American Journal of Physiology. Heart and Circulatory Physiology, 311(5), H1311–H1320.
Koopman, F. A., Chavan, S. S., Miljko, S., Grazio, S., Sokolovic, S., Schuurman, P. R., Mehta, A. D., Levine, Y. A., Faltys, M., Zitnik, R., Tracey, K. J., & Tak, P. P. (2016). Vagus nerve stimulation inhibits cytokine production and attenuates disease severity in rheumatoid arthritis. Proceedings of the National Academy of Sciences of the United States of America, 113(29), 8284–8289.
Liu, J. H., Chen, Y., Yuen, M., Zhen, Z., Chan, C. W., Lam, K. S., Tse, H. F., & Yiu, K. H. (2016). Incremental prognostic value of global longitudinal strain in patients with type 2 diabetes mellitus. Cardiovascular Diabetology, 15, 22.
Funding
This study was funded by an Oklahoma Shared Clinical and Translational Resources pilot grant (NIGMS IDeA-CTR U54-GM104938) to Stavros Stavrakis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no competing interests.
Human Subjects/Informed Consent Statement
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000. Informed consent was obtained from all patients for being included in the study.
Animal Studies
No animal studies were carried out by the authors for this article.
Additional information
Associate Editor Ana Barac oversaw the review of this article
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tran, N., Asad, Z., Elkholey, K. et al. Autonomic Neuromodulation Acutely Ameliorates Left Ventricular Strain in Humans. J. of Cardiovasc. Trans. Res. 12, 221–230 (2019). https://doi.org/10.1007/s12265-018-9853-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12265-018-9853-6