Abstract
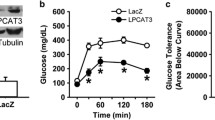
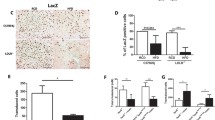
Lecithin:cholesterol acyltransferase (LCAT) is the key circulating enzyme responsible for high-density lipoprotein (HDL) cholesterol esterification, HDL maturation, and potentially reverse cholesterol transport. To further explore LCAT’s mechanism of action on lipoprotein metabolism, we employed adeno-associated viral vector (AAV) serotype 8 to achieve long-term (32-week) high level expression of human LCAT in hCETP;Ldlr+/− mice, and characterized the lipid profiles in detail. The mice had a marked increase in HDL cholesterol, HDL particle size, and significant reduction in low-density lipoprotein (LDL) cholesterol, plasma triglycerides, and plasma apoB. Plasma LCAT activity significantly increased with humanized substrate specificity. HDL cholesteryl esters increased in a fashion that fits human LCAT specificity. HDL phosphatidylcholines trended toward decrease, with no change observed for HDL lysophosphatidylcholines. Triglycerides reduction appeared to reside in all lipoprotein particles (very low-density lipoprotein (VLDL), LDL, and HDL), with HDL triglycerides composition highly reflective of VLDL, suggesting that changes in HDL triglycerides were primarily driven by the altered triglycerides metabolism in VLDL. In summary, in this human-like model for lipoprotein metabolism, AAV8-mediated overexpression of human LCAT resulted in profound changes in plasma lipid profiles. Detailed lipid analyses in the lipoprotein particles suggest that LCAT's beneficial effect on lipid metabolism includes not only enhanced HDL cholesterol esterification but also improved metabolism of apoB-containing particles and triglycerides. Our findings thus shed new light on LCAT’s mechanism of action and lend support to its therapeutic potential in treating dyslipidemia.






Similar content being viewed by others
References
Osuga, T., & Portman, O. W. (1971). Origin and disappearance of plasma lecithin: cholesterol acyltransferase. American Journal of Physiology, 220, 735–741.
Glomset, J. A. (1968). The plasma lecithins:cholesterol acyltransferase reaction. Journal of Lipid Research, 9, 155–167.
Francone, O. L., Gurakar, A., & Fielding, C. (1989). Distribution and functions of lecithin:cholesterol acyltransferase and cholesteryl ester transfer protein in plasma lipoproteins. Evidence for a functional unit containing these activities together with apolipoproteins A-I and D that catalyzes the esterification and transfer of cell-derived cholesterol. Journal of Biological Chemistry, 264, 7066–7072.
Rader, D. J., Ikewaki, K., Duverger, N., Schmidt, H., Pritchard, H., Frohlich, J., et al. (1994). Markedly accelerated catabolism of apolipoprotein A-II (ApoA-II) and high density lipoproteins containing ApoA-II in classic lecithin: cholesterol acyltransferase deficiency and fish-eye disease. The Journal of Clinical Investigation, 93, 321–330.
Fielding, C. J., & Fielding, P. E. (1995). Molecular physiology of reverse cholesterol transport. Journal of Lipid Research, 36, 211–228.
Applebaum-Bowden, D. (1995). Lipases and lecithin: cholesterol acyltransferase in the control of lipoprotein metabolism. Current Opinion in Lipidology, 6, 130–135.
Dobiasova, M., & Frohlich, J. J. (1999). Advances in understanding of the role of lecithin cholesterol acyltransferase (LCAT) in cholesterol transport. Clinica Chimica Acta, 286, 257–271.
Glomset, J. A. (1962). The mechanism of the plasma cholesterol esterification reaction: plasma fatty acid transferase. Biochimica et Biophysica Acta, 65, 128–135.
Kuivenhoven, J. A., Pritchard, H., Hill, J., Frohlich, J., Assmann, G., & Kastelein, J. (1997). The molecular pathology of lecithin:cholesterol acyltransferase (LCAT) deficiency syndromes. Journal of Lipid Research, 38, 191–205.
Calabresi, L., Pisciotta, L., Costantin, A., Frigerio, I., Eberini, I., Alessandrini, P., et al. (2005). The molecular basis of lecithin:cholesterol acyltransferase deficiency syndromes: a comprehensive study of molecular and biochemical findings in 13 unrelated Italian families. Arteriosclerosis, Thrombosis, and Vascular Biology, 25, 1972–1978.
Hovingh, G. K., Hutten, B. A., Holleboom, A. G., Petersen, W., Rol, P., Stalenhoef, A., et al. (2005). Compromised LCAT function is associated with increased atherosclerosis. Circulation, 112, 879–884.
Gjone, E. (1974). Familial lecithin:cholesterol acyltransferase deficiency—a clinical survey. Scandinavian Journal of Clinical and Laboratory Investigation. Supplementum, 137, 73–82.
Stokke, K. T., Bjerve, K. S., Blomhoff, J. P., Oystese, B., Flatmark, A., Norum, K. R., et al. (1974). Familial lecithin:cholesterol acyltransferase deficiency. Studies on lipid composition and morphology of tissues. Scandinavian Journal of Clinical and Laboratory Investigation. Supplementum, 137, 93–100.
Homma, S., Murayama, N., Yoshida, I., Kusano, E., Kuriki, K., Saito, K., et al. (2001). Marked atherosclerosis in a patient with familiar lecithin: cholesterol acyltransferase deficiency associated with end-stage renal disease and diabetes mellitus. American Journal of Nephrology, 21, 415–419.
Funke, H., von Eckardstein, A., Pritchard, P. H., Hornby, A. E., Wiebusch, H., Motti, C., et al. (1993). Genetic and phenotypic heterogeneity in familial lecithin: cholesterol acyltransferase (LCAT) deficiency. Six newly identified defective alleles further contribute to the structural heterogeneity in this disease. The Journal of Clinical Investigation, 91, 677–683.
Lambert, G., Sakai, N., Vaisman, B. L., Neufeld, E. B., Marteyn, B., Chan, C. C., et al. (2001). Analysis of glomerulosclerosis and atherosclerosis in lecithin cholesterol acyltransferase-deficient mice. Journal of Biological Chemistry, 276, 15090–15098.
Berard, A. M., Foger, B., Remaley, A., Shamburek, R., Vaisman, B. L., Talley, G., et al. (1997). High plasma HDL concentrations associated with enhanced atherosclerosis in transgenic mice overexpressing lecithin-cholesteryl acyltransferase. Nature Medicine, 3, 744–749.
Foger, B., Chase, M., Amar, M. J., Vaisman, B. L., Shamburek, R. D., Paigen, B., et al. (1999). Cholesteryl ester transfer protein corrects dysfunctional high density lipoproteins and reduces aortic atherosclerosis in lecithin cholesterol acyltransferase transgenic mice. Journal of Biological Chemistry, 274, 36912–36920.
Hoeg, J. M., Santamarina-Fojo, S., Berard, A. M., Cornhill, J. F., Herderick, E. E., Feldman, S. H., et al. (1996). Overexpression of lecithin:cholesterol acyltransferase in transgenic rabbits prevents diet-induced atherosclerosis. Proceedings of the National Academy of Sciences of the United States of America, 93, 11448–11453.
Zhang, A. H., Gao, S., Fan, J. L., Huang, W., Zhao, T. Q., & Liu, G. (2004). Increased plasma HDL cholesterol levels and biliary cholesterol excretion in hamster by LCAT overexpression. FEBS Letters, 570, 25–29.
Amar, M. J., Shamburek, R. D., Vaisman, B., Knapper, C. L., Foger, B., Hoyt, R. F., Jr., et al. (2009). Adenoviral expression of human lecithin-cholesterol acyltransferase in nonhuman primates leads to an antiatherogenic lipoprotein phenotype by increasing high-density lipoprotein and lowering low-density lipoprotein. Metabolism, 58, 568–575.
Vandenberghe, L. H., Wilson, J. M., & Gao, G. (2009). Tailoring the AAV vector capsid for gene therapy. Gene Therapy, 16, 311–319.
Nakai, H., Fuess, S., Storm, T. A., Muramatsu, S., Nara, Y., & Kay, M. A. (2005). Unrestricted hepatocyte transduction with adeno-associated virus serotype 8 vectors in mice. Journal of Virology, 79, 214–224.
Inagaki, K., Fuess, S., Storm, T. A., Gibson, G. A., McTiernan, C. F., Kay, M. A., et al. (2006). Robust systemic transduction with AAV9 vectors in mice: efficient global cardiac gene transfer superior to that of AAV8. Molecular Therapy, 14, 45–53.
Wang, Z., Zhu, T., Rehman, K. K., Bertera, S., Zhang, J., Chen, C., et al. (2006). Widespread and stable pancreatic gene transfer by adeno-associated virus vectors via different routes. Diabetes, 55, 875–884.
Gao, G., Lu, Y., Calcedo, R., Grant, R. L., Bell, P., Wang, L., et al. (2006). Biology of AAV serotype vectors in liver-directed gene transfer to nonhuman primates. Molecular Therapy, 13, 77–87.
Gao, G. P., Alvira, M. R., Wang, L., Calcedo, R., Johnston, J., & Wilson, J. M. (2002). Novel adeno-associated viruses from rhesus monkeys as vectors for human gene therapy. Proceedings of the National Academy of Sciences of the United States of America, 99, 11854–11859.
Chen, C. H., & Albers, J. J. (1982). Characterization of proteoliposomes containing apoprotein A-I: a new substrate for the measurement of lecithin: cholesterol acyltransferase activity. Journal of Lipid Research, 23, 680–691.
Kobori, K., Saito, K., Ito, S., Kotani, K., Manabe, M., & Kanno, T. (2002). A new enzyme-linked immunosorbent assay with two monoclonal antibodies to specific epitopes measures human lecithin-cholesterol acyltransferase. Journal of Lipid Research, 43, 325–334.
Albers, J. J., Chen, C. H., & Adolphson, J. L. (1981). Lecithin:cholesterol acyltransferase (LCAT) mass; its relationship to LCAT activity and cholesterol esterification rate. Journal of Lipid Research, 22, 1206–1213.
Pahl, M. V., Ni, Z., Sepassi, L., Moradi, H., & Vaziri, N. D. (2009). Plasma phospholipid transfer protein, cholesteryl ester transfer protein and lecithin:cholesterol acyltransferase in end-stage renal disease (ESRD). Nephrology, Dialysis, Transplantation, 24, 2541–2546.
Grove, D., & Pownall, H. J. (1991). Comparative specificity of plasma lecithin:cholesterol acyltransferase from ten animal species. Lipids, 26, 416–420.
Liu, M., Bagdade, J. D., & Subbaiah, P. V. (1995). Specificity of lecithin:cholesterol acyltransferase and atherogenic risk: comparative studies on the plasma composition and in vitro synthesis of cholesteryl esters in 14 vertebrate species. Journal of Lipid Research, 36, 1813–1824.
Kuksis, A., & Marai, L. (1967). Determination of the complete structure of natural lecithins. Lipids, 2, 217–224.
Adlof, R. (1991). Fractionation of egg and soybean phosphatidylcholines by silver resin chromatography. Journal of Chromatography, 538, 469–473.
Castelli, W. P., Doyle, J. T., Gordon, T., Hames, C. G., Hjortland, M. C., Hulley, S. B., et al. (1977). HDL cholesterol and other lipids in coronary heart disease. The cooperative lipoprotein phenotyping study. Circulation, 55, 767–772.
Havel, R. J. (1979). High-density lipoproteins, cholesterol transport and coronary heart disease. Circulation, 60, 1–3.
Assmann, G., & Gotto, A. M., Jr. (2004). HDL cholesterol and protective factors in atherosclerosis. Circulation, 109, III8–III14.
Nicholls, S. J., Tuzcu, E. M., Sipahi, I., Grasso, A. W., Schoenhagen, P., Hu, T., et al. (2007). Statins, high-density lipoprotein cholesterol, and regression of coronary atherosclerosis. Jama., 297, 499–508.
Amar, M. E., Santamarina-Fojo, S., Brewer, H. B., Jr., & Hoeg, J. M. (1997). Overexpression of human lecithin:cholesterol acyltransferase in cholesterol-fed rabbits: LDL metabolism and HDL metabolism are affected in a gene dose-dependent manner. Metabolism, 38, 568–575.
Brousseau, M. E., Kauffman, R. D., Herderick, E. E., Demosky, S. J., Jr., Evans, W., Marcovina, S., et al. (2000). LCAT modulates atherogenic plasma lipoproteins and the extent of atherosclerosis in lecithin cholesterol acyltransferase transgenic mice. Journal of Biology, 20, 450–458.
Norum, K. R., Glomset, J. A., Nichols, A. V., & Forte, T. (1971). Plasma lipoproteins in familial lecithin: cholesterol acyltransferase deficiency: physical and chemical studies of low and high density lipoproteins. The Journal of Clinical Investigation, 50, 1131–1140.
Acknowledgments
We thank our colleagues Bo Wang and Christian Nunes for support of the tail vein injection, Beth Murphy for coordinating mice shipment, and Yong-hua Zhu for the pathology report.
Author information
Authors and Affiliations
Corresponding authors
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 43 kb)
Rights and permissions
About this article
Cite this article
Chen, Z., Chu, D., Castro-Perez, J.M. et al. AAV8-Mediated Long-Term Expression of Human LCAT Significantly Improves Lipid Profiles in hCETP;Ldlr+/− Mice. J. of Cardiovasc. Trans. Res. 4, 801–810 (2011). https://doi.org/10.1007/s12265-011-9309-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12265-011-9309-8