Abstract
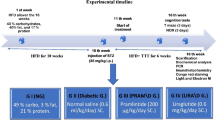
Hyperhomocysteinemia (Hhcy) is an independent risk factor for Alzheimer’s disease (AD), and insulin-resistance is commonly seen in patients with Hhcy. Liraglutide (Lir), a glucagon-like peptide that increases the secretion and sensitivity of insulin, has a neurotrophic or neuroprotective effect. However, it is not known whether Lir ameliorates the AD-like pathology and memory deficit induced by Hhcy. By vena caudalis injection of homocysteine to produce the Hhcy model in rats, we found here that simultaneous administration of Lir for 2 weeks ameliorated the Hhcy-induced memory deficit, along with increased density of dendritic spines and up-regulation of synaptic proteins. Lir also attenuated the Hhcy-induced tau hyperphosphorylation and Aβ overproduction, and the molecular mechanisms involved the restoration of protein phosphatase-2A activity and inhibition of β- and γ-secretases. Phosphorylated insulin receptor substrate-1 also decreased after treatment with Lir. Our data reveal that Lir improves the Hhcy-induced AD-like spatial memory deficit and the mechanisms involve the modulation of insulin-resistance and the pathways generating abnormal tau and Aβ.






Similar content being viewed by others
References
Glenner GG, Wong CW. Alzheimer’s disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem Biophys Res Commun 1984, 120: 885–890.
Grundke-Iqbal I, Iqbal K, Tung YC, Quinlan M, Wisniewski HM, Binder LI. Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology. Proc Natl Acad Sci U S A 1986, 83: 4913–4917.
Lopera F, Ardilla A, Martinez A, Madrigal L, Arango-Viana JC, Lemere CA, et al. Clinical features of early-onset Alzheimer disease in a large kindred with an E280A presenilin-1 mutation. JAMA 1997, 277: 793–799.
Zhou SJ, Zhang LG, Chen HM, Li JY, Li R, Zhang XM, et al. Prevalence and clinical-demographic correlates of hyperhomocysteinemia in inpatients with bipolar disorder in a Han Chinese population. Psychiatry Res 2018, 259: 364–369.
Zhang CE, Tian Q, Wei W, Peng JH, Liu GP, Zhou XW, et al. Homocysteine induces tau phosphorylation by inactivating protein phosphatase 2A in rat hippocampus. Neurobiol Aging 2008, 29: 1654–1665.
Wei W, Liu YH, Zhang CE, Wang Q, Wei Z, Mousseau DD, et al. Folate/vitamin-B12 prevents chronic hyperhomocysteinemia-induced tau hyperphosphorylation and memory deficits in aged rats. J Alzheimers Dis 2011, 27: 639–650.
Zhang CE, Wei W, Liu YH, Peng JH, Tian Q, Liu GP, et al. Hyperhomocysteinemia increases beta-amyloid by enhancing expression of gamma-secretase and phosphorylation of amyloid precursor protein in rat brain. Am J Pathol 2009, 174: 1481–1491.
Chai GS, Jiang X, Ni ZF, Ma ZW, Xie AJ, Cheng XS, et al. Betaine attenuates Alzheimer-like pathological changes and memory deficits induced by homocysteine. J Neurochem 2013, 124: 388–396.
Li B, Xi X, Roane DS, Ryan DH, Martin RJ. Distribution of glucokinase, glucose transporter GLUT2, sulfonylurea receptor-1, glucagon-like peptide-1 receptor and neuropeptide Y messenger RNAs in rat brain by quantitative real time RT-PCR. Brain Res Mol Brain Res 2003, 113: 139–142.
Larsen PJ, Tang-Christensen M, Holst JJ, Orskov C. Distribution of glucagon-like peptide-1 and other preproglucagon-derived peptides in the rat hypothalamus and brainstem. Neuroscience 1997, 77: 257–270.
Nakade Y, Tsukamoto K, Pappas TN, Takahashi T. Central glucagon like peptide-1 delays solid gastric emptying via central CRF and peripheral sympathetic pathway in rats. Brain Res 2006, 1111: 117–121.
Abbas T, Faivre E, Holscher C. Impairment of synaptic plasticity and memory formation in GLP-1 receptor KO mice: interaction between type 2 diabetes and Alzheimer’s disease. Behav Brain Res 2009, 205: 265–271.
During MJ, Cao L, Zuzga DS, Francis JS, Fitzsimons HL, Jiao X, et al. Glucagon-like peptide-1 receptor is involved in learning and neuroprotection. Nat Med 2003, 9: 1173–1179.
Lee CH, Yoo KY, Ryu PD, Park JH, Choi JH, Kim S, et al. Decreased glucagon-like peptide-1 receptor immunoreactivity in the dentate granule cell layer from adult in the gerbil hippocampus. Cell Mol Neurobiol 2011, 31: 345–350.
Juhl CB, Hollingdal M, Sturis J, Jakobsen G, Agerso H, Veldhuis J, et al. Bedtime administration of NN2211, a long-acting GLP-1 derivative, substantially reduces fasting and postprandial glycemia in type 2 diabetes. Diabetes 2002, 51: 424–429.
Chang AM, Jakobsen G, Sturis J, Smith MJ, Bloem CJ, An B, et al. The GLP-1 derivative NN2211 restores beta-cell sensitivity to glucose in type 2 diabetic patients after a single dose. Diabetes 2003, 52: 1786–1791.
Li L, Zhang ZF, Holscher C, Gao C, Jiang YH, Liu YZ. (Val(8)) glucagon-like peptide-1 prevents tau hyperphosphorylation, impairment of spatial learning and ultra-structural cellular damage induced by streptozotocin in rat brains. Eur J Pharmacol 2012, 674: 280–286.
Gault VA, Holscher C. GLP-1 agonists facilitate hippocampal LTP and reverse the impairment of LTP induced by beta-amyloid. Eur J Pharmacol 2008, 587: 112–117.
McClean PL, Holscher C. Liraglutide can reverse memory impairment, synaptic loss and reduce plaque load in aged APP/PS1 mice, a model of Alzheimer’s disease. Neuropharmacology 2014, 76 Pt A: 57–67.
Ahren B, Pacini G. Dose-related effects of GLP-1 on insulin secretion, insulin sensitivity, and glucose effectiveness in mice. Am J Physiol 1999, 277: E996–E1004.
Vorhees CV, Williams MT. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc 2006, 1: 848–858.
Zaqout S, Kaindl AM. Golgi-Cox staining step by step. Front Neuroanat 2016, 10: 38.
Giltay EJ, Hoogeveen EK, Elbers JM, Gooren LJ, Asscheman H, Stehouwer CD. Insulin resistance is associated with elevated plasma total homocysteine levels in healthy, non-obese subjects. Atherosclerosis 1998, 139: 197–198.
Luo L, Ma J, Li Y, Hu Z, Jiang C, Cai H, et al. Cystatin C induces insulin resistance in hippocampal neurons and promotes cognitive dysfunction in rodents. Neurosci Bull 2018, 34: 543–545.
Arnold SE, Arvanitakis Z, Macauley-Rambach SL, Koenig AM, Wang HY, Ahima RS, et al. Brain insulin resistance in type 2 diabetes and Alzheimer disease: concepts and conundrums. Nat Rev Neurol 2018, 14: 168–181.
MacDonald PE, El-Kholy W, Riedel MJ, Salapatek AM, Light PE, Wheeler MB. The multiple actions of GLP-1 on the process of glucose-stimulated insulin secretion. Diabetes 2002, 51 Suppl 3: S434–S442.
Hoyer S, Muller D, Plaschke K. Desensitization of brain insulin receptor. Effect on glucose/energy and related metabolism. J Neural Transm Suppl 1994, 44: 259–268.
Henneberg N, Hoyer S. Desensitization of the neuronal insulin receptor: a new approach in the etiopathogenesis of late-onset sporadic dementia of the Alzheimer type (SDAT)? Arch Gerontol Geriatr 1995, 21: 63–74.
Schachter M, Raziel A, Friedler S, Strassburger D, Bern O, Ron-El R. Insulin resistance in patients with polycystic ovary syndrome is associated with elevated plasma homocysteine. Hum Reprod 2003, 18: 721–727.
Golbahar J, Aminzadeh MA, Kassab SE, Omrani GR. Hyperhomocysteinemia induces insulin resistance in male Sprague-Dawley rats. Diabetes Res Clin Pract 2007, 76: 1–5.
Copps KD, White MF. Regulation of insulin sensitivity by serine/threonine phosphorylation of insulin receptor substrate proteins IRS1 and IRS2. Diabetologia 2012, 55: 2565–2582.
Hoehn KL, Hohnen-Behrens C, Cederberg A, Wu LE, Turner N, Yuasa T, et al. IRS1-independent defects define major nodes of insulin resistance. Cell Metab 2008, 7: 421–433.
Talbot K, Wang HY. The nature, significance, and glucagon-like peptide-1 analog treatment of brain insulin resistance in Alzheimer’s disease. Alzheimers Dement 2014, 10: S12–S25.
Talbot K, Wang HY, Kazi H, Han LY, Bakshi KP, Stucky A, et al. Demonstrated brain insulin resistance in Alzheimer’s disease patients is associated with IGF-1 resistance, IRS-1 dysregulation, and cognitive decline. J Clin Invest 2012, 122: 1316–1338.
Wang HY, Bakshi K, Frankfurt M, Stucky A, Goberdhan M, Shah SM, et al. Reducing amyloid-related Alzheimer’s disease pathogenesis by a small molecule targeting filamin A. J Neurosci 2012, 32: 9773–9784.
Bomfim TR, Forny-Germano L, Sathler LB, Brito-Moreira J, Houzel JC, Decker H, et al. An anti-diabetes agent protects the mouse brain from defective insulin signaling caused by Alzheimer’s disease- associated Abeta oligomers. J Clin Invest 2012, 122: 1339–1353.
Ji W, Chen X, Lv J, Wang M, Ren S, Yuan B, et al. Liraglutide exerts antidiabetic effect via PTP1B and PI3K/Akt2 signaling pathway in skeletal muscle of KKAy mice. Int J Endocrinol 2014, 2014: 312452.
Sontag E, Nunbhakdi-Craig V, Sontag JM, Diaz-Arrastia R, Ogris E, Dayal S, et al. Protein phosphatase 2A methyltransferase links homocysteine metabolism with tau and amyloid precursor protein regulation. J Neurosci 2007, 27: 2751–2759.
Qi L, Chen Z, Wang Y, Liu X, Liu X, Ke L, et al. Subcutaneous liraglutide ameliorates methylglyoxal-induced Alzheimer-like tau pathology and cognitive impairment by modulating tau hyperphosphorylation and glycogen synthase kinase-3beta. Am J Transl Res 2017, 9: 247–260.
Kojro E, Gimpl G, Lammich S, Marz W, Fahrenholz F. Low cholesterol stimulates the nonamyloidogenic pathway by its effect on the alpha -secretase ADAM 10. Proc Natl Acad Sci U S A 2001, 98: 5815–5820.
Cai H, Wang Y, McCarthy D, Wen H, Borchelt DR, Price DL, et al. BACE1 is the major beta-secretase for generation of Abeta peptides by neurons. Nat Neurosci 2001, 4: 233–234.
McGowan E, Pickford F, Kim J, Onstead L, Eriksen J, Yu C, et al. Abeta42 is essential for parenchymal and vascular amyloid deposition in mice. Neuron 2005, 47: 191–199.
Iwatsubo T, Odaka A, Suzuki N, Mizusawa H, Nukina N, Ihara Y. Visualization of A beta 42(43) and A beta 40 in senile plaques with end-specific A beta monoclonals: evidence that an initially deposited species is A beta 42(43). Neuron 1994, 13: 45–53.
Lee MS, Kao SC, Lemere CA, Xia W, Tseng HC, Zhou Y, et al. APP processing is regulated by cytoplasmic phosphorylation. J Cell Biol 2003, 163: 83–95.
Acknowledgements
This work was supported by the National Key R&D Program of China, National Basic Research Development Program of the Ministry of Science and Technology of China (2016YFC1305800), the National Natural Science Foundation of China (31730035, 91632305, and 81721005), and the Integrated Innovation Team for Major Human Disease Program of Tongji Medical College, Huazhong University of Science and Technology, China.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors claim that there are no conflicts of interest.
Rights and permissions
About this article
Cite this article
Zhang, Y., Xie, JZ., Xu, XY. et al. Liraglutide Ameliorates Hyperhomocysteinemia-Induced Alzheimer-Like Pathology and Memory Deficits in Rats via Multi-molecular Targeting. Neurosci. Bull. 35, 724–734 (2019). https://doi.org/10.1007/s12264-018-00336-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12264-018-00336-7