Abstract
Industrialization leads to the entry of diverse xenobiotic compounds into the environment. One such compound is paracetamol (APAP), which is emerging as a pharmaceutical and personal care pollutant (PPCP). In this study, the APAP degrading bacterium was isolated by enrichment culture method from the sewage sample. The microscopy, biochemical, and 16S rRNA gene sequence analyzed the isolate PPY-2, which belongs to Bacillus licheniformis, and GenBank assigned accession number MN744328. Physiological and batch culture degradation studies have indicated that the strain involved in the degradation of APAP. The optimum pH for degradation of the PPY-2 was 7.7, whereas the temperature was 25 °C, agitation speed was 142 rpm, and concentration of APAP was 621 mg/L reported, and the optimum temperatures were 42 °C and 32 °C, respectively. Biomass kinetic was studied at optimal physical conditions, which suggested that the specific growth rate (μ) was 721 mg/L. The GC–MS chromatogram peaks have detected metabolites, viz., oxalic acid, 2-isopropyl-5-methyl cyclohexanone, and phenothiazine. The study confirmed that Bacillus licheniformis strain PPY-2 exhibits metabolic potential to biodegradation APAP and can be further deployed in bioremediation.
Similar content being viewed by others
1 Introduction
Pharmaceutical and personal care products (PPCPs) are contaminants that develop unexpectedly with pervasive chemical substances, such as pharmaceutical products, non-steroidal anti-inflammatory drugs (NSAIDs), antibiotics, hormones, etc. They have been used in animal husbandry, healthcare, agriculture, and healthcare (Daughton and Ternes 2001, 2009; Mohapatra et al. 2016). However, they reach the aquatic environment in various ways (Wu et al. 2012), and domestic waste is the main route through which it enters the atmosphere. The main entrance through which it enters the atmosphere is domestic waste and sewage treatment plants (Chang et al. 2011). The debris found in the sewage treatment plants is very consistent in household sewage, small- and medium-sized industrial sewage, agricultural product waste, hospital sewage, etc. In addition, they are composed of human and animal excretions and non-metabolized products. However, in a few cases, almost 60–95% of the active substances, such as NSAIDs, in the form of capsules and in tablet form, etc., are excreted by the patients as the metabolic value differs from species to species and from person to person (Chopra and Kumar 2018). Other similar studies have indicated the degradation of various compounds using isolated strains, such as the biodegradation of ibuprofen by Bacillus siamensis strain DSI-1 (Chopra and Kumar 2022), acid red 14 from synthetic wastewater by advanced oxidation process with artificial neural network and fuzzy regression (Asadollahfardi et al. 2022), marine bacterial consortium used for degradation of cold-adapted oil/PAH-degrading (Crisafi et al. 2016), hexavalent molybdenum by Serratia sp. strain MIE2 (Halmi et al. 2016), cyanide removal by Serratia marcescens strain AQ07 (Karamba et al. 2016), pyrene degradation by Achromobacter denitrificans ASU-035 (Mawad et al. 2016), and more recently surfactant degradation by Pseudomonas and Bacillus spp. and also by microbial consortia containing Alcaligenes spp., Citrobacter spp., reported by Arora et al., (2022).
Pharmaceutical use drugs have given rise to a class of hazardous compounds, "emerging contaminants" (ECs), and they are classified as "pharmaceutical pollutants". These compounds have varied chemical structures and bioactivity and are grouped among xenobiotics (Rivera-Utrilla et al. 2013; aus der Beek et al. 2016). Their revival strategy from water has been described in a review by aus der Beek et al. (2016). NSAIDs are a subset of PPCPs; it consists of medicines commonly prescribed to relieve pain, lower fever, stop blood clots, and reduce inflammation. The essential non-steroidal distinguishes these capsules from steroids (Żur et al. 2018a, b). This was first studied on steroids in the 1960s, but there was little known about their steroid use. NSAIDs commonly treat acute or ongoing problems: mild pain, arthritis, inflammation, tissue damage, migraine, extreme arthritis, pain (menstrual pain), bone pain, post-operative pain due to Parkinson's disease, muscle wrinkles, and pain, painful accidents together with the flu, also known as the colon, kidney, macular edema, etc. are effective in controlling surgical pain after tooth extraction (Newton et al. 1982; Song and Chen 2001; Du et al. 2016). Paracetamol (acetaminophen, APAP), in combination with chemicals like N-(4-hydroxyphenyl) ethanamide and N-(4-hydroxyphenyl) acetamide, can form a monocyclic NSAID. First developed in 1877, and until now, this is the most widely used drug worldwide and also one of all significant medicines indexed by the World Health Organization (WHO). Over the past 2 decades, the expansion in production, consumption, and transportation of APAP had concluding its occurrence in many water bodies, landfills, and sewage treatment plants. On the other hand, it has obstructed the marine environment (Marchlewicz et al. 2015). APAP was reported among 29 countries in surface water, groundwater, and tap or drinking water, in environmental sources with an average concentration (0.161 g/L), and a maximum (230 g/L) (aus der Beek et al. 2016). It acts as a micropollutant, and its presence has been increased through various food chains and led to chronic health risks and toxic effects (Gu 2020). It is among the top ten drugs consumed by countries worldwide. In 2016, the United States consumed around 49-kilo metric tons, followed by China in the second place with 34.6-kilo metric tons and Europe with 48.4-kilo metric tons (Debortoli et al. 2021). APAP actively treats a wide range of symptoms such as flu, cough, cold, fever, allergy, pain, and sleep disorders; therefore, during the Coronavirus disease-2019 (COVID-19) disaster, this molecule was included as a significant component of the therapeutic plans and was widely consumed globally (Poddar et al. 2022). As a result, the consumption rate in Greece increased by 198% during the pandemic era (Galani et al. 2021). A similar pattern was found throughout the world among several countries during the waves of the COVID-19 epidemic from 2019 to 2021. According to the most recent estimate of APAP demand, the output is increasing worldwide. The continuous increase in APAP usage has led to demand for their increased production. China and India are the leading nations worldwide for APAP production, accounting for more than 70% of the global APAP supply (Park et al. 2021). Other countries like the United States, Australia, European Union, Hong Kong, Thailand, and Indonesia also play a significant role in its production. The massive amount of APAP produced by companies increased the amounts of discharged effluents from the process, resulting in environmental effects, and their permitted level is problematic for international environmental organizations for pharmaceutical pollutants, including APAP (Becht et al. 2021; Poddar et al. 2022).Biodegradation is a less inexpensive method of degradation of APAP through biological entities. This can convert APAP into CO2 and H2O as the end product of degradation (Hesnawi et al. 2014). It also detects numerous errors that can affect APAP. In our experience, the first studied microbe has been assigned the genus Penicillium sp., which can degrade APAP to 4-aminophenol and other acetate compounds (Hart and Orr 1975). Rhodococcus can break down to 4-aminophenol, hydroquinone, catechol, Delftia tsuruhatensis, and the genus Pseudomonas spp. convert it to hydroquinone (De-Gusseme et al. 2011; Akay and Tezel 2020), and by Pseudomonas strain PrS10 isolated from pharmaceutical effluents (Poddar et al. 2022). Hydroquinone has been converted to 1,2-dioxygenase, hydroquinone, and acyclic products by Arthrobacter sp., Phanerochaete chrysosporium, and Burkholderia cepacia AC1100 (Takenaka et al. 2003; Paolis et al. 2013). However, microorganisms could degrade APAP into methylated N-acetyl-p-benzoquinone-imine (NAPIQ) and 3-hydroxy-acetaminophen (Marchlewicz et al. 2015) and such intermediates metabolites produced upon degradation are even more toxic than APAP (Chopra and Kumar 2020a). More recently, Fernandes et al. (2021) reviewed the fate and bioremediation of pharmaceuticals by aquatic environments. Other similar studies have indicated the involvement of the isolated strains in biodegradation of diverse types of compounds, such as polycyclic aromatic hydrocarbons (PAHs), phenanthrene by Comamonas testosteroni strain-T (Olukanni et al. 2022), and also PAH by fungus Podoscypha elegans Strain FTG4 (Agrawal et al. 2021), pharmaceutical wastes in treated sewage effluents by Bacillus subtilis 1556WTNC (Al-Gheethi and Ismail 2014), methylene blue by textile effluent biotreatment by Acinetobacter pitti (Ogunlaja et al. 2020) etc.
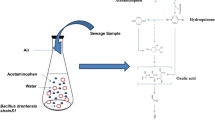
Therefore, this study was focused on the isolation of bacterial strains that has the potential to degrade APAP from wastewater samples by enrichment culture method. During the degradation study, APAP acts as a source of carbon and energy in the metabolic activity of tolerant isolates. The biodegradation experiments were planned using Design-Expert® software-based optimization. Other bacteria having degradation potential of APAP have been harvested, and Haldane growth model was used for the analysis of the kinetics of biomass growth and the results analyzed. Morphological, physiological, and molecular studies are used for strain characterization utilizing strain inherent properties based on phenotypic and genotypic characteristics. Finally, 16S rRNA gene sequencing was used for molecular identification of the strain up to their species. The intermediate metabolite compounds produced upon biodegradation have been identified through gas-chromatography–mass spectroscopy (GC–MS), PathPred, and a degradation pathway was proposed.
2 Materials and methods
2.1 Chemical and collection of samples
In this study, the chemicals and medium components were purchased from Merck, Qualigens, HiMedia (Mumbai, India), and Acetaminophen (99% purity, Sigma-Aldrich USA). The wastewater samples were taken from the pulp and paper industry in Yamunanagar, Haryana, India. The samples were collected in sterile containers and stored in refrigerated condition. The collected sample has biological oxygen demand (BOD) of more than 1200, and the sample was black and has a foul smell. The suspended particles and debris were removed and filtered with Whatman filter paper #1.
2.2 Isolation and screening of APAP degrading isolates
Initial biodegradation experiments were set up in 250 mL conical flasks having Bushnell Haas Medium (BHM, HiMedia, India) as used in the study by Chopra and Kumar (2020b) based on an enrichment culture method for isolation of potential degrading bacteria. Further, the screening of degrading bacterial isolates was performed up to 1500 mg/L concentration of APAP. The degradation of APAP was observed with the UV-spectrophotometer at OD265 (Kesur et al. 2012). The degradation percentage (R) was calculated by Eq. (1)
where C0 is the absorbance at the initial amount of APAP; Ct is the absorbance after incubation of the sample after time 't'.
2.3 Identification and characterization of APAP degrading isolates
Morphological, biochemical, and molecular characterization of APAP degrading isolates was performed as per the standard methods. Biochemical tests were conducted per the protocols mentioned in Bergey's Manual of Systematic Bacteriology (2012) and the laboratory manual by Cappuccino and Sherman (2019). Gram staining of bacteria was done using a staining kit, and biochemical characterization was performed using HiBacillus identification kit KB013 (HiMedia, Mumbai, India). Further, the alkaline lysis method was used to isolate the genomic DNA of bacterial isolate (Wilson 1987), and after that, polymerase chain reaction (PCR) amplification was as per the conditions mentioned by Chopra and Kumar (2020b) in their other study. Using 16S rRNA universal gene primer 27F 5′- (AGAGTTTGATCMTGGCTCAG)-3′ and 1492R 5′ (CGGTTACCTTGTTACGACTT)3′. 16S rRNA gene sequencing was performed with Sanger sequencing. Finally, the evolutionary strain relationship was inferred using the Neighbor-Joining (NJ) method, and phylogenetic analyses were performed with Molecular Evolutionary Genetic Analysis software (MEGA 11.0, https://megasoftware.net/) (Kumar et al. 2016).
The experimental data of biodegradation of APAP were further subjected to the Haldane growth kinetics model, which is the modified version of the Monod kinetics model (Halden et al. 1997). Best fit was analyzed with Origin 2017 software (Deschenes LA, of Texas A 2000). Haldane growth model kinetic analysis Eq. (2)
where 'μ' is the specific growth rate of individual strain; μmax is the maximum specific growth rate of strain after time 't'; 'S' is the amount of paracetamol (mg/L); 'Ks' is half-saturation constant (mg/L) and 'Ki' is inhibition constant (mg/L). Further, the yield coefficient of the individual strain and linear regression were used by accessing Eq. (3)
where 'X' and 'X0' are the biomass of strain S1 at the time 't' and the initial amount of biomass strain S1 (mg/L), respectively, and 'S' and 'S0' are the paracetamol concentration after time 't' and the initial amount of paracetamol (mg/L).
2.4 Biodegradation study and identification of degradation metabolites
To understand the biodegradation kinetics, the experimental design, optimization of experiments, and statistical analysis of APAP degradation by isolated trains were performed to know the best fit of physical conditions, such as temperature, pH, and rpm. Hence, the kinetic study of degrading biomass was conducted using the method given in our other study (Chopra and Kumar 2020b) and other similar studies (Zhang et al. 2013; Palma et al. 2021; Żur et al. 2018b).
FTIR and GC–MS-based identification of metabolites after biodegradation of the sample was conducted as per the standard method given by the instrument manufacturer, and consequently, the samples for this analysis were prepared with the method described by Chopra and Kumar (2020b). Finally, after investigation, a web-based tool, PathPred, was used to predict the pathway of degradation of the metabolites mediated by the strain of this study. Quantification of degrading metabolites mediated by the isolated strain was analyzed by comparing the percent relative peak area. Identification of the compounds was made by the comparison of retention time (RT in minutes) and mass spectra data with National Institute of Standards and Technology (NIST, Gaithersburg, MD, USA), and WILEY library data of the GC–MS system.
3 Results and discussion
3.1 Isolation and screening of APAP degrading bacterial isolates
The enrichment technique isolated four bacterial isolates from the pulp and paper industry wastewater during the initial degradation. Finally, PPY2 was selected for other studies due to its tolerance of up to 1500 mg/L of APAP. This study indicated that the isolates have authentic APAP degrading activity and were explored for further characterization. Zhang et al. (2013) observed that members of the genera Stenotrophomonas and Pseudomonas were involved in paracetamol in aerobic conditions. In bath culture study of the organisms f1, f2, and fg-2 led to complete degradation of paracetamol of 400, 2500, and 2000 mg/L at concentrations or less concentration, respectively. In co-culture, degrade 4000 mg/L degraded and 87.1% of paracetamol. Further, two key metabolites of the paracetamol biodegradation pathway, 4-aminophenol and hydroquinone, were observed in the degradation culture. Another study characterized Pseudomonas aeruginosa strain HJ1012 as involved in the biodegradation of paracetamol (Hu et al. 2013). Martinez-Hernández et al. (2016) envisaged the role of sorption and biodegradation for removing Acetaminophen, carbamazepine, caffeine, naproxen, and sulfamethoxazole in soil system and kinetic analysis of degradation studied. Baratpour and Moussavi (2018) studied enhanced biodegradation of Acetaminophen by H2O2 stimulated up-flow fixed-bed bioreactor (UFBR). Żur et al. (2018a) studied the micropollutant paracetamol and ibuprofen for their toxicity and genetic mechanism in degradation. In another study, Żur et al. (2018b) characterized Pseudomonas moorei KB4 strain for paracetamol degradation and used 50 mg/L of paracetamol. Later, degradation products, p-aminophenol, and hydroquinone, were detected and thereby proposed the degradation pathway of paracetamol. Further, this study can be helpful in the treatment of wastewater contamination of paracetamol. In co-metabolic conditions, bacterial strains utilize glucose. Additionally, the degradation is influenced by many environmental factors such as pH, temperature, and heavy metals. Also, when degradation is mediated by immobilized cells of Pseudomonas moorei KB4, by this strain, the degradation rate is enhanced (Surma et al. 2021).
3.2 Characterization of APAP degrading bacterial isolates
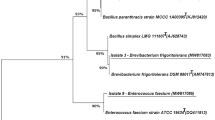
Through morphological and microscopic characterization, it was found that the PPY2 isolate was rod-shaped has Gram-positive nature, and biochemical test results were conducted, which showed the diverse metabolic potential of the isolates (Table 1). DNA was isolated from them, and PCR was performed using 16S rRNA gene sequence. After PCR amplification, about 1500 bp amplified 16S rRNA gene sequence was obtained. The partial sequences of 16S rDNA were obtained after the Sanger DNA sequencing method. There was no chimera detected in the generated sequence after analyzing with DECIPHER online analysis tool, and BLAST analysis was performed for the sequence of PPY-2 isolate. The result has shown that the isolate belonging to the Bacillus licheniformis was closely matched to the Bacillus licheniformis strain CIIRC/M1 16S ribosomal RNA gene and the partial sequence with a 98.22% similarity level. Further, this sequence was submitted to NCBI, and accession number MN744328 was provided for the Bacillus licheniformis strain PPY-2 16S ribosomal RNA gene, a partial sequence. Finally, a Neighbor-Joining (NJ) phylogenetic tree was constructed for 14 strains selected from the NCBI database having similarity detected with our strain, and APAP biodegradation strain PPY-2 (Fig. 1). This resulted in 15 nucleotide sequences, and codon positions at 1st + 2nd + 3rd + noncoding position have been seen. The ambiguous positions were further removed for each sequence pair by employing the pairwise deletion option, and of 1559 positions in the final dataset were detected, which were used for evolutionary analyses. Lebaron et al. (1998) described the phenotypic and genetic diversity for microbial characterization and showed their utility in microbial identification.
3.3 Experimental design, optimization study, and statistical analysis of biodegradation by PPY-2 isolate
The best physical condition for the degradation of APAP mediated by the strains PPY-2, the parameters A, pH; B, temperature; C, agitation speed; and D, the concentration of APAP, were used. The BBD model has resulted in the form of a matrix having experiments (Table 2) using the response surface study. The quadratic design model was used to generate the matrix. The experiments were performed in the laboratory with the predicted set for the matrix. The APAP degradation percentage value was subjected to the matrix and further processed by the BBD-quadratic model. Variance analysis (ANOVA) was performed with the BBD-quadratic model for degradation by the isolated bacterial strain PPY-2 (Table 3). The significant nature of the model gives the value to the sum of squares of 23,347.36, degree of freedom (df) of 14 for each, mean square of 1667.67, F value of 23.36, and p values of < 0.0001. Further, the report was generated based on predicted value with reference to actual values. The contour plots and 3D plots have been developed between two parameters: time vs percent degradation of APAP (represented as AB vs percent degradation). Similarly, contour plots and 3D plots were used to analyze all other physical parameters (Fig. 2). Such 3D plots have shown the effects of a physical factor on the degradation of APAP by the strain PPY-2. Further, the response surface values have been fitted in the equation, as given below
where Y is the predicted response (APAP degradation), A (pH), B (temperature), C (APAP concentration), and D (agitation speed) were the independent variables. They finally decided the fate of biodegradation by the isolated bacterial strain. Finally, the optimal values for the parameters, viz., pH, temperature, agitation speed, and concentration of APAP, were observed for PPY-2 were almost 7.7, 25 °C, 142 rpm, and the software optimized 621 mg/L.
3.4 Batch culture degradation study
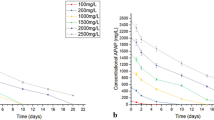
This research was carried out under optimal conditions in a batch reactor of 20 L supplemented with PPY-2 to degrade APAP in wastewater. A distinct chemical is generally found in wastewater. APAP degrading batch growth kinetics and biomass data were used for analysis. Such plots were utilized in this study to determine the specific growth rate (μ) for the starting concentration of APAP. When the concentration of APAP was raised, the specific growth rate of microorganisms rose. However, after attaining Vmax, further deterioration begins as APAP concentration increases (Fig. 3). APAP degradation data subjected to the Haldane growth model using Origin 2017 software. This model suggested that the specific growth rate (μ) for the strain PPY-2 was 721 mg/L concentration (Table 4). Further, the μmax and half-saturation constant were calculated.
3.5 Metabolite analysis and pathway prediction
The distinctive bands of degradation of APAP by Bacillus licheniformis strain PPY-2 were detected using FTIR in CIL, DCRUST. Due to the low dosage of APAP utilized in this work, weak spectra were seen. An sp2 C–H stretch band at 3098.98/cm indicates the alkane portion. Several bands in the range stated the existence of aromatic rings. The CO band may be found at 2070.97/cm. At 1621.56/cm, characteristic C=C vibration stretch bands were observed. Between 3681 and 4000/cm, overtone combo bands develop. Hydroxide, carbon–hydrogen, and nitrogen–hydrogen stretching caused vibrations between 4000 and 2500/cm. Hydroxide stretching has a broad range of 3700–3600/cm, whereas NH stretching is between 3400 and 3300/cm. R–OH ranges from 2500 to 3000. Para-substituted aromatic compounds have an out-of-plane CH bending at 736.23/cm and phenol O–H stretching at 3550–3500.
Many metabolites were formed by APAP biodegradation and were investigated by GC–MS in hexane extract. The gas chromatogram revealed 76 peaks, each representing a separate metabolite eluting close together (retention times of 17.709–51.510). The library of gas chromatogram peaks is generated by mass spectroscopy. GC–MS analysis findings demonstrate that no APAP residue was left in the batch culture. This implies that PPY-2 uses APAP as an energy source and can remove APAP from wastewater. After 48 h, the color of the media shifted from white to brown and then to black after 5 days. The degrading response is now underway. It is critical to understand the metabolic mechanism of APAP degradation by PPY-2 for the degradation investigation. The APAP catabolic pathway metabolites were definitively identified as oxalic acid, 2-isopropyl-5-methyl cyclohexanone, and phenothiazine. Many studies have indicated the characterization of bacteria for paracetamol as a carbon and energy source for isolated strains of bacteria. There metabolic pathways for paracetamol biodegradation have also been suggested (Gusseme et al. 2011; Zhang et al. 2013; Karaman et al. 2016) Such as isolated strains from the genera Stenotrophomonas and Pseudomonas (Zhang et al. 2013), Pseudomonas aeruginosa based biodegradation by the isolated strain from activated sludge (Karaman et al. (2016). In the metabolic pathway for paracetamol biodegradation, the first metabolites 4-aminophenol and hydroquinone produced (Zhang et al. 2013; Karaman et al. 2016), and Pseudomonas aeruginosa strains STB2 and STB4 degraded 79.4% and 88.4% of paracetamol (with a concentration of 3000 mg/L), respectively, observed in 120 h (Abdullah et al. 2018) and the similar results obtained in this study. After batch incubation, intermediates compound obtained during the degradation of APAP were identified by GC–MS analysis. Published reports on APAP confirmed the derivatives obtained by the mass spectra library. The degradation of APAP was analyzed by GC–MS, leading to identifying compounds such as oxalic acid and 2-isopropyl-5-methyl cyclohexanone based on the query submitted to the PathPred server. The predicted pathway was similar to that of Chopra and Kumar (2020b).
Palma et al. (2021) studied the biodegradation of paracetamol by Gram-positive bacteria. Alobaidi et al. (2021) explained the biodegradation of emerging pharmaceuticals by membrane bioreactor from domestic wastewater. Hasan et al. (2021) described the biodegradation potential of salicylic acid, acetaminophen, and ibuprofen through bacteria isolated from a full-scale drinking water biofilter. Grignet et al. (2022) reviewed the environmental implications of medicines as emerging contaminants, which need to be given attention. They suggested the medicines as the emerging contaminant that need a special attention in the current scenario. Water of medicines might have a prolonged effect on the ecosystem, and metabolites from medicines disrupt microbial dynamics in the ecosystem. Therefore, in this direction, bioremediation act as the efficient end eco-friendly technology, which can be used in water treatment to reduce the load of emerging contaminants. Muras et al. (2021) explained the importance of Bacillus licheniformis in various sectors like producing bioactive compounds, food, aquaculture, biomedicine, pharmaceutical industry, bioremediation, biomineralization, etc. By considering the isolated strain further applications, this study can be further deployed in bioremediation programs or industrial biotechnology. More recently, Rios-Miguel et al. (2022) described the application of uncharacterized amidases enzyme in paracetamol degradation, and they isolated two Pseudomonas sp. isolates from wastewater treatment plants. They tolerated 200 mg/L of paracetamol in 10 h, releasing 4-aminophenol as a degradation intermediate. Pseudomonas strain PrS10 recovered from pharmaceutical effluents, which has highest paracetamol degradation potential of 96.37% in 7 days (Poddar et al. 2022). Hence, such study, further, helps to boost our knowledge of microorganisms’ role in the biodegradation of pharmaceutical compounds and the diversity of amidase enzymes that may have a role in paracetamol degradation in wastewater treatment plants (WWTPs).
4 Conclusion
Paracetamol is a common drug pain reliever and is used in treating fever in humans. But due to its continuous use, it is causing contaminates in different water bodies by the release into the environmental ecosystem. Therefore, there is a need for decontamination of the biological source contaminated with APAP. Hence bioremediation technology can be employed for the remediation of such compounds from the environment. Bacillus licheniformis strain PPY-2 was reported in the present work, and this strain successfully degraded the APAP in the batch culture study. This study indicated that the isolated strain could grow on an APAP-containing medium, and the strain mediated the transformation of this compound.
Availability of data and materials
The 16S rRNA gene sequence data of the paracetamol degrading Bacillus licheniformis strain PPY-2 were deposited to National Centre of Biotechnology Information (NCBI) GenBank with accession number MN744328.
Code availability
Not applicable.
References
Abdullah QY, Edrees WH, AL-Kaf AG, Naji KM (2018) Biodegradation of paracetamol by native bacterial strains isolated from Yemeni pharmaceutical wastewater plant in Sana’a. Chron Pharm Sci 2:512–522
Agrawal N, Barapatre A, Shahi MP et al (2021) Biodegradation pathway of polycyclic aromatic hydrocarbons by ligninolytic fungus Podoscypha elegans strain FTG4 and phytotoxicity evaluation of their metabolites. Environ Process 8:1307–1335. https://doi.org/10.1007/s40710-021-00525-z
Akay C, Tezel U (2020) Biotransformation of Acetaminophen by intact cells and crude enzymes of bacteria: A comparative study and modelling. Sci Total Environ 703:134990. https://doi.org/10.1016/j.scitotenv.2019.134990
Al-Gheethi AAS, Ismail N (2014) Biodegradation of pharmaceutical wastes in treated sewage effluents by Bacillus subtilis 1556WTNC. Environ Process 1:459–481. https://doi.org/10.1007/s40710-014-0034-6
Alobaidi RAK, Ulucan-Altuntas K, Mhemid RKS et al (2021) Biodegradation of emerging pharmaceuticals from domestic wastewater by membrane bioreactor: the effect of solid retention time. Int J Environ Res Public Health 18:3395
Asadollahfardi G, Afsharnasab M, Rasoulifard MH et al (2022) Predicting of acid red 14 removals from synthetic wastewater in the advanced oxidation process using artificial neural networks and fuzzy regression. Rend Fis Acc Lincei 33:115–126. https://doi.org/10.1007/s12210-021-01043-8
aus der Beek T, Weber FA, Bergmann A, Hickmann S, Ebert I, Hein A et al (2016) Pharmaceuticals in the environment–Global occurrences and perspectives. Environ Toxicol Chem 35:823–835. https://doi.org/10.1002/etc.3339
Baratpour P, Moussavi G (2018) The accelerated biodegradation and mineralization of Acetaminophen in the H2O2-stimulated upflow fixed-bed bioreactor (UFBR). Chemosphere 210:1115–1123
Becht A, Schollmayer C, Monakhova Y, Holzgrabe U (2021) Tracing the origin of paracetamol tablets by near-infrared, mid-infrared, and nuclear magnetic resonance spectroscopy using principal component analysis and linear discriminant analysis. Anal Bioanal Chem 413:3107–3118. https://doi.org/10.1007/s00216-021-03249-z
Cappuccino J, Sherman N (2019) Microbiology: a laboratory manual, Ed. 2019. Benjamin-Cummings Publishing Company, New York
Chang H, Wan Y, Wu S et al (2011) Occurrence of androgens and progestogens in wastewater treatment plants and receiving river waters: comparison to estrogens. Water Res 45:732–740. https://doi.org/10.1016/j.watres.2010.08.046
Chopra S, Kumar D (2018) Pharmaceuticals and personal care products (PPCPs) as emerging environmental pollutants: toxicity and risk assessment. In: Gahlawat SK, Duhan JS, Salar RK et al (eds) Advances in animal biotechnology and its applications. Springer, Singapore, pp 337–353
Chopra S, Kumar D (2020a) Biodegradation and kinetic analysis of Acetaminophen with co-culture of bacterial strains isolated from sewage wastewater. Curr Microbiol 77:3147–3157
Chopra S, Kumar D (2020b) Characterization, optimization and kinetics study of acetaminophen degradation by Bacillus drentensis strain S1 and waste water degradation analysis. Bioresour Bioprocess 7:25. https://doi.org/10.1186/s40643-020-0297-x
Chopra S, Kumar D (2022) Characterization and biodegradation of ibuprofen by Bacillus siamensis strain DSI-1 isolated from wastewater. Rend Fis Acc Lincei 33:643–652. https://doi.org/10.1007/s12210-022-01085-6
Crisafi F, Giuliano L, Yakimov MM et al (2016) Isolation and degradation potential of a cold-adapted oil/PAH-degrading marine bacterial consortium from Kongsfjorden (Arctic region). Rend Fis Acc Lincei 27:261–270. https://doi.org/10.1007/s12210-016-0550-6
Daughton CG, Ternes TA (2001) Pharmaceuticals and care products in the environment. American Chemical Society, Washington, DC, p 791. https://doi.org/10.1021/bk-2001-0791
Daughton CG, Ternes TA (2009) Pharmaceuticals and personal care products in the environment: agents of subtle change? Environ Toxicol 28:2663–2670. https://doi.org/10.1021/bk-2001-0791
De Gusseme B, Vanhaecke L, Verstraete W, Boon N (2011) Degradation of acetaminophen by Delftia tsuruhatensis and Pseudomonas aeruginosa in a membrane bioreactor. Water Res 45:1829–1837. https://doi.org/10.1016/j.watres.2010.11.040
Debortoli C, Lan R, Lafont J et al (2021) Paracetamol misusing to dental pain: a case-report and recommandations for treatment. J Oral Med Oral Surg 27:57
Deschenes LA, of Texas A (2000) Origin 6.0: Scientific data analysis and graphing software origin lab corporation (formerly Microcal Software, Inc.). www.originlab.com. Commercial price: 595.Academicprice: 446
Grignet RDS, Barros MGA, Panatta AAS, Bernal SPF, Ottoni JR, Passarini MRZ, Gonçalves CCS (2022) Medicines as an emergent contaminant: the review of microbial biodegration potential. Folia Microbiol 67(2):157–174. https://doi.org/10.1007/s12223-021-00941-6
Du J, Mei C-F, Ying G-G, Xu M-Y (2016) Toxicity Thresholds for diclofenac, acetaminophen and ibuprofen in the water flea daphnia magna. Bull Environ Contam Toxicol 97:84–90. https://doi.org/10.1007/s00128-016-1806-7
Fernandes JP, Almeida CMR, Salgado MA et al (2021) Pharmaceutical compounds in aquatic environments—occurrence, fate and bioremediation prospective. Toxics 9:257
Galani A, Alygizakis N, Aalizadeh R et al (2021) Patterns of pharmaceuticals use during the first wave of COVID-19 pandemic in Athens, Greece as revealed by wastewater-based epidemiology. Sci Total Environ 798:149014
Gu JD (2020) On environmental biotechnology of bioremediation. Appl Environ Biotech 5(2):28–33. https://doi.org/10.26789/AEB.2020.02.002
Halden RU, Halden BG, Dwyer DF (1997) A kinetic analysis of dioxin degradation in bioaugmented soils. In: Abstr Annu Meet Am Soc Microbiol, poster Q-381
Halmi MI, Abdullah SR, Wasoh H, Johari WL, Ali MS, Shaharuddin NA, Shukor MY (2016) Optimization and maximization of hexavalent molybdenum reduction to Mo-blue by Serratia sp. strain MIE2 using response surface methodology. Rend Fis Acc Lincei 27(4):697–709
Hart A, Orr DL (1975) The degradation of paracetamol (4-hydroxyacetanilide) and other substituted acetanilides by a Penicillium species. Antonie Van Leeuwenhoek 41:239–247. https://doi.org/10.1007/BF02565059
Hasan M, Alfredo K, Murthy S, Riffat R (2021) Biodegradation of salicylic acid, Acetaminophen and ibuprofen by bacteria collected from a full-scale drinking water biofilter. J Environ Manage 295:113071
Hesnawi R, Dahmani K, Al-Swayah A et al (2014) Biodegradation of municipal wastewater with local and commercial bacteria. Procedia Eng 70:810–814. https://doi.org/10.1016/J.PROENG.2014.02.088
Hu J, Zhang LL, Chen JM, Liu Y (2013) Degradation of paracetamol by Pseudomonas aeruginosa strain HJ1012. J Environ Sci Health A Tox Hazard Subst Environ Eng 48:791–799. https://doi.org/10.1080/10934529.2013.744650
Karaman R, Khamis M, Abbadi J et al (2016) Paracetamol biodegradation by activated sludge and photocatalysis and its removal by a micelle–clay complex, activated charcoal, and reverse osmosis membranes. Environ Technol 37:2414–2427
Karamba KI, Ahmad SA, Zulkharnain A et al (2016) Optimisation of biodegradation conditions for cyanide removal by Serratia marcescens strain AQ07 using one-factor-at-a-time technique and response surface methodology. Rend Fis Acc Lincei 27:533–545. https://doi.org/10.1007/s12210-016-0516-8
Kesur BR, Salunkhe VR, Magdum CS (2012) Development and validation of UV spectrophotometric method for simultaneous estimation of ibuprofen and famotidine in bulk and formulated tablet dosage form. Int J Pharm Pharm Sci 4:271–274
Kumar S, Stecher G, Tamura K (2016) MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol 33(7):1870–1874. https://doi.org/10.1093/molbev/msw054
Lebaron P, Ghiglione J-F, Fajon C et al (1998) Phenotypic and genetic diversity within a colony morphotype. FEMS Microbiol Lett 160:137–143
Marchlewicz A, Guzik U, Wojcieszyńska D (2015) Over-the-counter monocyclic non-steroidal anti-inflammatory drugs in environment—sources, risks, biodegradation. Water Air Soil Pollut 2015:226. https://doi.org/10.1007/s11270-015-2622-0
Martinez-Hernández V, Meffe R, López SH, de Bustamante I (2016) The role of sorption and biodegradation in the removal of Acetaminophen, carbamazepine, caffeine, naproxen and sulfamethoxazole during soil contact: a kinetics study. Sci Total Environ 559:232–241
Mawad AMM, Hesham AEL, Mostafa YM et al (2016) Pyrene degrading Achromobacter denitrificans ASU-035: growth rate, enzymes activity, and cell surface properties. Rend Fis Acc Lincei 27:557–563. https://doi.org/10.1007/s12210-016-0521-y
Mohapatra S, Huang CH, Mukherji S, Padhye LP (2016) Occurrence and fate of pharmaceuticals in WWTPs in India and comparison with a similar study in the United States. Chemosphere 159:526–535. https://doi.org/10.1016/j.chemosphere.2016.06.047
Muras A, Romero M, Mayer C, Otero A (2021) Biotechnological applications of Bacillus licheniformis. Crit Rev Biotechnol 41:609–627
Newton JF, Kuo C-H, Gemborys MW et al (1982) Nephrotoxicity of p-aminophenol, a metabolite of Acetaminophen, in the Fischer 344 rat. Toxicol Appl Pharmacol 65:336–344. https://doi.org/10.1016/0041-008X(82)90017-5
Ogunlaja A, Nwankwo IN, Omaliko ME et al (2020) Biodegradation of methylene blue as an evidence of synthetic dyes mineralization during textile effluent biotreatment by Acinetobacter pittii. Environ Process 7:931–947. https://doi.org/10.1007/s40710-020-00443-6
Olukanni OD, Famuyiwa T, Oyenuga S et al (2022) Enhanced biodegradation of phenanthrene by Comamonas testosteroni strain T in the presence of limiting concentration of triton x–100. Environ Process 9:57. https://doi.org/10.1007/s40710-022-00608-5
Palma TL, Magno G, Costa MC (2021) Biodegradation of paracetamol by some gram-positive bacterial isolates. Curr Microbiol 78:2774–2786
Paolis MR, Lippi D, Guerriero E et al (2013) Biodegradation of a-, b-, and c-Hexachlorocyclohexane by Arthrobacter fluorescens and Arthrobacter giacomelloi. Appl Biochem Biotechnol 2013:170. https://doi.org/10.1007/s12010-013-0147-9
Park J, Kelly MA, Kang JX et al (2021) Production of active pharmaceutical ingredients (APIs) from lignin-derived phenol and catechol. Green Chem 23:7488–7498
Poddar K, Sarkar D, Chakraborty D et al (2022) Paracetamol biodegradation by Pseudomonas strain PrS10 isolated from pharmaceutical effluents. Int Biodeterior Biodegrad 175:105490
Rios-Miguel AB, Smith GJ, Cremers G et al (2022) Microbial paracetamol degradation involves a high diversity of novel amidase enzyme candidates. Water Res X 16:100152
Rivera-Utrilla J, Sanchez-Polo M, Ferro-Garcıa MA, Prados-Joya G, Ocampo-Perez R (2013) Pharmaceuticals as emerging contaminants and their removal from water. A Review. Chemosphere 93:1268–1287. https://doi.org/10.1016/j.chemosphere.2013.07.059
Song H, Chen TS (2001) p-Aminophenol-induced liver toxicity: Tentative evidence of a role for Acetaminophen. J Biochem Mol Toxicol 15:34–40. https://doi.org/10.1002/1099-0461(2001)15:1%3c34::AID-JBT4%3e3.0.CO;2-U
Surma R, Wojcieszynska D, Karcz J, Guzik U (2021) Effect of Pseudomonas moorei KB4 “Cells” immobilisation on their degradation potential and tolerance towards paracetamol. Molecules 26:820
Takenaka S, Okugawa S, Kadowaki M et al (2003) The metabolic pathway of 4-Aminophenol in Burkholderia sp. strain AK-5 Differs from that of aniline and aniline with C-4 substituents. Appl Environ Microbiol 69:541–5413. https://doi.org/10.1128/AEM.69.9.5410-5413.2003
Wu S, Zhang L, Chen J (2012) Paracetamol in the environment and its degradation by microorganisms. Appl Microbiol Biotechnol 96:875–884. https://doi.org/10.1007/s00253-012-4414-4
Zhang L, Hu J, Zhu R, Zhou Q, Chen J (2013) Degradation of paracetamol by pure bacterial cultures and their microbial consortium. Appl Microbiol Biotechnol 97(8):3687–4369. https://doi.org/10.1007/s00253-012-4170-5
Żur J, Piński A, Marchlewicz A et al (2018a) Organic micropollutants paracetamol and ibuprofen–-toxicity, biodegradation, and genetic background of their utilization by bacteria. Environ Sci Pollut Res 25:21498–21524. https://doi.org/10.1007/s11356-018-2517-x
Żur J, Wojcieszyńska D, Hupert-Kocurek K, Marchlewicz A, Guzik U (2018b) Paracetamol—toxicity and microbial utilization. Pseudomonas moorei KB4 as a case study for exploring degradation pathway. Chemosphere 206:192–202. https://doi.org/10.1016/j.chemosphere.2018.04.179
Acknowledgements
The authors acknowledge the sample analysis for FTIR at Central Instrumentation Laboratory (CIL), DCRUST Murthal Sonepat India, DNA sequencing at Eurofins Genomics India Pvt Ltd, Advanced Instrumentation Research Facility (AIRF), JNU New Delhi, India for GC–MS analysis. The Author, S. Chopra, wishes to thank UGC New Delhi India for providing a research assistantship in the form of an RGNF fellowship.
Funding
There is no external funding received to carry out this research. The authors wish to thank the Department of Biotechnology, DCRUST Murthal, Sonepat, India, for providing the necessary facilities to carry out this research.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by SC and DK. The first draft of the manuscript was written by SC and all authors commented on previous versions of the manuscript. DK has supervised this research.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest.
Ethical approval for research involving human participants and/or animals
Not applicable.
Informed consent has been provided
Not applicable, as this study does not involve any human/animal study.
Consent to participate
Both authors were involved in the manuscript.
Consent for publication
The authors have consent for the publication and approved the final draft of the manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chopra, S., Kumar, D. Characterization and biodegradation of paracetamol by biomass of Bacillus licheniformis strain PPY-2 isolated from wastewater. Rend. Fis. Acc. Lincei 34, 491–501 (2023). https://doi.org/10.1007/s12210-023-01140-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12210-023-01140-w