Abstract
Objectives
Due to its industrial application and frequent use as a coating material for food containers, bisphenol A (4,4′-isopropylidenediphenol, BPA) is present in abundance in our environment. Data on intake levels of BPA are limited in preadolescent children in Japan. This study was designed to help us better understand the current state of BPA exposure in children in Japan.
Methods
We followed first graders (n = 104) attending school in a Tokyo suburb from 1998 until the sixth grade (2003), during which time we collected a total of three morning urine samples. Urinary BPA was analyzed using high-performance liquid chromatography isotope-dilution tandem mass spectrometry.
Results
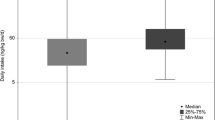
Ninety-four children were followed for 5 years. Median urinary BPA level was 2.66 ng/mg creatinine (CRE) (range 0.9–38.9) at first grade (1998), 1.52 ng/mg CRE (0.4–11.8) at third grade (2000), and 0.66 ng/mg CRE (0.2–8.5) at sixth grade (2003), showing a significant decrease in urinary BPA levels over a 5-year follow-up study (p < 0.001). No significant difference was seen between boys and girls at each grade.
Conclusions
Urinary levels of BPA were relatively low throughout the study period; however, as the study progressed, we observed a significant decline in levels, the reason behind which is not yet clear.
Similar content being viewed by others
Introduction
Bisphenol A (4,4′-isopropylidenediphenol, BPA) is widely used as an ingredient of polycarbonate (PC) and epoxy resins and is often used for polyethylene terephthalate (PET) bottles, cans, and instant-noodle and box-lunch containers. In Japan, dishes made of PC are widely used for serving elementary school lunches. Concern has therefore been expressed that elution of BPA from school lunch dishes is a source of exposure in children through food [1, 2]. Nevertheless, production of these items has recently increased, from approximately 440,000 tons in 2001 to approximately 530,000 tons in 2005 [3].
The estrogenic actions of BPA have been primarily observed in an MCF-7 human breast cancer cell proliferation study [4] and many in vitro assays [5–7]. Moreover, its in vivo effects on the endocrine system at the microgran level have been reported in F344 and Sprague-Dawley (SD) rats and mice and include cell proliferation and c-fos expression in the uterus and vagina [8], increased prostate weight [9], increased size of the preputial glands, decreased size of the epididymis, and lower sperm production [10]. As fetuses and newborns, whose organs are in the developmental stage, are particularly susceptible to the effects of endocrine disrupters [11–14], a human epidemiological survey that focused on the association between endocrine disrupters and congenital anomalies was carried out [15, 16]. To ascertain the level of exposure to BPA, BPA levels were measured in the blood of children with hypospadia and on umbilical cord blood of mothers or pregnant women of children with other congenital anomalies [13, 16–18], and the association with BPA was estimated based on those data. To our knowledge, however, no study has investigated BPA levels in biological samples from healthy children, and the state of BPA exposure among Japanese children is unknown.
Here, to better understand BPA exposure in Japanese children, we measured urinary BPA levels in school children using high-performance liquid chromatography isotope-dilution tandem mass spectrometry (LC-MS/MS) and investigated changes in exposure over time.
Methods
Subjects and sample collection
First graders (n = 105) attending a school in a suburb of Tokyo whose guardians provided written consent were followed from 1998 to 2003. Spot urine was sampled three times in the same children, once each in February during the first, third, and sixth grade. At the same time, a simple life-style survey was performed using a questionnaire, and physical measurements were taken. Participation in the survey was obtained from 104 of 105 first graders in the first year of the survey, so 104 children were followed up thereafter. The study was approved by the ethics review committee of Showa University School of Medicine.
Analytical methods
We stored obtained urine samples at −80°C until completion of the survey, at which time all samples were analyzed simultaneously. We partially modified the method of Itoh et al. [19] to measure urinary BPA. Using 200-μl urine samples, we deconjugated by treatment with beta-glucuronidase/sulfatase, followed by solid-phase extraction and analysis using isotope-dilution LC-MS/MS. This method allowed the lower limit of detection (LOD) of 0.5 ng/ml with good reproducibility. We used BPA-d16 as a surrogate. In addition, we measured creatinine levels using an autoanalyzer (550EXPRESS, Chiron Corporation) to correct urinary levels. Urinary BPA concentration (ng/ml) was divided by corresponding individual urinary creatinine concentration to correct for variability in urine dilution.
Statistical analysis
Data for the 94 children able to be followed for 5 years were analyzed. For measurement values below the LOD, we assigned a value equal to the LOD divided by the square root of 2 (0.35 ng/ml) [20]. Moreover, we used creatinine (CRE)-corrected values for all numerical values used in the analysis. Urinary BPA levels were log-transformed to improve normality of the distribution. Differences in urinary BPA levels and anthropometric factors between boys and girls in each grade were tested using Student’s t test. The linear mixed model was used to compare urinary BPA levels between the first, third, and sixth grade while adjusting for body mass index (BMI). All p values reported are two-sided, and significance level was set at p < 0.01. All statistical analyses were performed with the SAS software version 9.1 (SAS Institute Inc., Cary, NC, USA).
Results
This was a follow-up study of 104 first graders, but the number of subjects decreased to 103 (99.0%) in third grade and 94 (90.4%) in sixth grade, leaving 94 children (90.4%) able to be followed for the full 5 years: 53 boys and 41 girls. Among the ten students who could not be followed, five transferred to other schools and five did not provide consent. Subject characteristics are shown in →→ 1. No significant difference in anthropometric factors was seen between boys and girls.
BPA (0.5 ng/ml) was detected in 94/94 (100%) children during first grade, 91/94 (97%) in third grade, and 81/94 (86%) in sixth grade. As shown in Fig. 1, median urinary BPA levels were 2.66 ng/mg CRE (range 0.9–38.9) in first grade (1998), 1.52 ng/mg CRE (range 0.4–11.8) in third grade (2000), and 0.66 ng/mg CRE (range 0.2–8.5) in sixth grade (2003), showing a significant decrease in BPA levels with increasing grade (p < 0.001). No significant difference in BPA level was seen between boys and girls at any grade.
In addition, this data was a comparable result regardless of the presence of the CRE correction.
Discussion
Many reports have described a variety of adverse health effects of BPA in children. Further, in animal reproduction studies, effects on F1 and F2 generations have also been seen, such as reproductive system effects and behavioral abnormalities in animal experiments using mice and rats [8, 9, 11, 21, 22].
Moreover, human epidemiological studies have suggested the possibility of an association with a variety of disorders, including effects on the reproductive system, such as hypospadia, undescended testes, and endometriosis [15, 19, 23, 24]; neural development and behavior [12, 25]; sex hormones [12, 24]; and allergic conditions [26]. In terms of the risk of BPA to infants and children, an expert panel at the Center for the Evaluation of Risks to Human Reproduction of the National Toxicology Program in the United States recently expressed “some concern that exposure to Bisphenol A causes neural and behavioral effects” and “minimal concern that exposure to Bisphenol A potentially causes accelerations in puberty” [27]. In Japan, however, a paucity of epidemiological survey data remains [28], and no reports on exposure in children have appeared.
Clearance of BPA from the body is relatively fast, and its terminal half-life is 3–6 h [29]. Because the major metabolites glucuronide conjugates of BPA are rapidly excreted into urine within 24 h [30, 31], it has been suggested that urinary BPA levels represent a good indicator of daily exposure [17]. On this basis, we investigated the exposure of children in Japan using urinary BPA levels in a group of school children.
Determination of urinary BPA levels was done using LC-MS/MS [19] on account of its highly reliable identification. Various techniques are used to analyze BPA levels in biological samples, including instrumental analysis [32–35] and enzyme-linked immunosorbent assay (ELISA) [36]. However, these assays are hindered by a variety of problems, including complexity (e.g., sample cleanup and derivatization) and low sensitivity. For example, ELISA is easy to use but is hampered by yielding BPA levels that are somewhat high. Further, the possibility of cross-reaction with BPA-related substances has been noted [27, 37]. Here, therefore, we decided to measure BPA levels using LC-MS/MS [19], a highly sensitive technique with high reproducibility.
The frequency rate of urinary BPA in this group was high, at 100% in the first grade, 97% in the third grade, and 86% in the sixth grade. The range after CRE correction was 0.2–38.9 ng/mg CRE (median 1.5). Conversion based on daily BPA intake (daily representative value for CRE excretion in children: 23.3 mg/kg per day [38]) gave 0.005–0.9 μg/kg per day (median 0.035). These values are roughly consistent with those reported previously in Japanese university students, namely, 0.02 μg/kg per day [39] and 0.1–11.9 ng/mg CRE [2], as well as the 3.0 μg/g CRE (0.3–54.3) [40], 4.3 μg/g CRE (3.6–5.1) [41] reported in children in the United States, and 1.2 μg/kg per day intake from canned foods by children aged 4–6 years estimated by the Scientific Committee on Food (SCF) in Europe [42]. However, the present values were much lower than the BPA tolerable daily intake (TDI) of 0.01 mg/kg body weight/day proposed by the SCF [42]. Nevertheless, given reports of adverse effects [11, 43, 44] at doses even approximately 1/1,000 those at which adverse effects are reported to occur in conventional animal studies, we consider the present results to be meaningful.
In terms of changes in BPA levels over time, a significant (p < 0.001) decrease was observed in the ranking of first grade (1998) > third grade (2000) > sixth grade (2003) (Fig. 1). This study was a follow-up of 104 children, but an additional 21 children entered the school when the subjects were third graders and a further 23 in the sixth grade, for whom consent was also obtained and measurements were performed. Additional analysis incorporating this extra data gave a median level of 1.58 ng/mg CRE (range 0.4–21.0) in the third and 0.66 ng/mg CRE (range 0.05–8.5) in the sixth grade, again showing a tendency toward a significant decrease in CRE levels year by year (Kruskal–Wallis rank test, p < 0.001). This consistent decrease may be explained as follows:
First, food-container-related regulations might be involved. In Japan, 1998, growing public concern over problems caused by endocrine-disrupting chemicals prompted major concerned companies to make efforts to confront potential problems by substituting the PC coating of beverage and food cans with a polyethylene terephtalate (PET) film patch or painting with a resin low in BPA elution. This process was almost finished by 2001. Further, the ratio of canned food intake to total food intake gradually decreased after 1995 [45]. Matsumoto et al. [33] measured urinary BPA levels in university students in 1992 and 1999 and reported that 1992 levels were about 2.2 times higher than the 1999 levels. One possible reason they gave for this decrease was a decrease in the use of beverage cans coated with PC. Using dishes made of PC in elementary school lunches was acknowledged as a problem, and thus replacing them with polypropylene dishes, which do not contain BPA, was promoted. In 1998, 40.1% of schools were using PC dishes, decreasing to as little as 10.2% in 2003 [45], and thus prompting a nationwide substitution of PC resin for polypropylene dishes. The school in our study shifted to polypropylene dishes in March 2002, when the subjects were in fifth grade, and items containing BPA have not been used at the school since. BPA was detected in 64 of 200 (32%) school lunch dishes subjected to elution testing of dishes used up to the fourth grade (unpublished data), with BPA levels under elution conditions stipulated in the Food Sanitation Law, namely, elution-solvent water, 4% acetic acid, 20% ethanol, and N-heptan, of 1.0–67.0 ppb (unpublished data). BPA was not detected in dishes used after the change. Given that the amount of BPA in dishes made of PC should not be more than 550 ppm (material standard) and not more than 2.5 ppm (elution standard) [46], elution from dishes actually used was below the reference values. Nevertheless, we consider that the subjects in this study were orally exposed to BPA from school lunch dishes almost every day up to the fourth grade and that exposure via school lunch dishes was subsequently eliminated.
It has been hypothesized that children aged 1–6 years may be exposed to greater levels of BPA than older or younger generations, as they eat a relatively larger amount of food per unit of body weight than others, and dishes of PC resin that might elute BPA are commonly used at mealtime. This result is based on an estimation of exposure made using the Monte Carlo technique, which integrates data from all conceivable exposure sources, including air, drinking water, meals (dishes and food), and soil [45]. Calafat et al. [41] measured urinary BPA in 2003–2004 and reported that the levels measured highest in 6- to 11-year-olds, and the second highest in 12- to 19-year-olds, followed by subjects aged 20 years or older. Higher concentrations of BPA in younger children may be explained by their higher level of food consumption and air inhalation. The subjects in our study were aged 6–12 years and seemed to ingest the average amount of food for their age bracket considering their nutritional requirements (1,379.4 ± 377.4 g/day, 7–14 years old [47]). Furthermore, our study was a follow-up study conducted on the same subjects as had previously been observed, and thus extreme changes in food preference were negligible.
In addition, Wolff et al. [39] measured urinary BPA levels in girls aged 6–8 years old and reported that urinary BPA levels were significantly lower in girls with a BMI at or above the 85th reference percentile compared with girls with a lower BMI. According to our data, however, no significant relationship was found between urinary BPA level or BMI, height, or body weight of children in the same grade, so we consider that any effect of physical size was negligible.
It is not fully understood how BPA affects the metabolisms of elementary school children of varying ages. Maximum glomerular filtration volume in the kidneys is attained 1 year after birth, and renal tubular function related to toxic substance excretion is said to reach the adult level at about age 2 [48]. From this information, there seems to be no difference in the levels of BPA excretion during the elementary school years (6–12 years old). However, as we did not simultaneously investigate urinary BPA levels among children of different grades or ages, no definite conclusion could be reached. Regardless of the subject’s age, similar exposure levels of BPA were expected to result in similar excretion levels in the urine.
In our 5-year follow-up study (1998–2003) of the same group of elementary school children, urinary BPA levels were 0.2–38.9 ng/mg CRE and were by no means high, even while school lunch dishes made of PC were being used. We also found that subject’s urinary BPA levels decreased significantly with increasing school grade. Although a cause cannot be identified, contributing candidates may include the social climate, including changes in school lunch dishes and regulations concerning the use of PC containers for food and beverages and coating agents.
References
Brotons JA, Olea-Serrano MF, Villalobos M, Pedraza V, Olea N. Xenoestrogens released from lacquer coatings in food cans. Environ Health Perspect. 1995;103(6):608–12.
Ouchi K, Watanabe S. Measurement of bisphenol A in human urine using liquid chromatography with multi-channel coulometric electrochemical detection. J Chromatogr B Analyt Technol Biomed Life Sci. 2002;780(2):365–70.
METI. Yearbook of chemical industry statistics. Research and Statistics Department, Economic and Industrial Policy Bureau Ministry of Economy, Trade and Industry, editor. Tokyo: Research Institute of Economy, Trade and Industry; 2006 (in Japanese).
Krishnan AV, Stathis P, Permuth SF, Tokes L, Feldman D. Bisphenol-A: an estrogenic substance is released from polycarbonate flasks during autoclaving. Endocrinology. 1993;132(6):2279–86.
Harris RM, Waring RH, Kirk CJ, Hughes PJ. Sulfation of “estrogenic” alkylphenols and 17beta-estradiol by human platelet phenol sulfotransferases. J Biol Chem. 2000;275(1):159–66.
Iso T, Watanabe T, Iwamoto T, Shimamoto A, Furuichi Y. DNA damage caused by bisphenol A and estradiol through estrogenic activity. Biol Pharm Bull. 2006;29(2):206–10.
Kurosawa T, Hiroi H, Tsutsumi O, Ishikawa T, Osuga Y, Fujiwara T, et al. The activity of bisphenol A depends on both the estrogen receptor subtype and the cell type. Endocr J. 2002;49(4):465–71.
Steinmetz R, Mitchner NA, Grant A, Allen DL, Bigsby RM, Ben-Jonathan N. The xenoestrogen bisphenol A induces growth, differentiation, and c-fos gene expression in the female reproductive tract. Endocrinology. 1998;139(6):2741–7.
Nagel SC, vom Saal FS, Thayer KA, Dhar MG, Boechler M, Welshons WV. Relative binding affinity-serum modified access (RBA-SMA) assay predicts the relative in vivo bioactivity of the xenoestrogens bisphenol A and octylphenol. Environ Health Perspect. 1997;105(1):70–6.
vom Saal FS, Cooke PS, Buchanan DL, Palanza P, Thayer KA, Nagel SC, et al. A physiologically based approach to the study of bisphenol A and other estrogenic chemicals on the size of reproductive organs, daily sperm production, and behavior. Toxicol Ind Health. 1998;14(1-2):239–60.
Howdeshell KL, Hotchkiss AK, Thayer KA, Vandenbergh JG, vom Saal FS. Exposure to bisphenol A advances puberty. Nature. 1999;401(6755):763–4.
Kishi R, Sata F, Saijo Y, Kurahashi N, Kato S, Nakajima S, et al. Exposure to endocrine disrupting chemicals and children’s health: problems in epidemiological studies. Nippon Eiseigaku Zasshi. 2006;61(1):19–31 (in Japanese).
Sakurai K, Mori C. Fetal exposure to endocrine disruptors. Nippon Rinsho. 2000;58(13):2508–13 (in Japanese).
Wilson NK, Chuang JC, Morgan MK, Lordo RA, Sheldon LS. An observational study of the potential exposures of preschool children to pentachlorophenol, bisphenol-A, and nonylphenol at home and daycare. Environ Res. 2007;103(1):9–20.
Hiroi H, Tsutsumi O, Takeuchi T, Momoeda M, Ikezuki Y, Okamura A, et al. Differences in serum bisphenol A concentrations in premenopausal normal women and women with endometrial hyperplasia. Endocr J. 2004;51(6):595–600.
Ikezuki Y, Tsutsumi O, Takai Y, Kamei Y, Taketani Y. Determination of bisphenol A concentrations in human biological fluids reveals significant early prenatal exposure. Hum Reprod. 2002;17(11):2839–41.
Fujimaki K, Arakawa C, Yoshinaga J, Watanabe C, Serizawa S, Imai H, et al. Estimation of intake level of bisphenol A in Japanese pregnant women based on measurement of urinary excretion level of the metabolite. Nippon Eiseigaku Zasshi. 2004;59(4):403–8 (in Japanese).
Hirahara F, Sumiyoshi Y, Yamanaka M, Endo M, Ishikawa H, Sugawara T, et al. Fetal exposure to endocrine disruptors during human pregnancy in Japan. International symposium on environmental endocrine disrupters: November 26–28, 2002.
Itoh H, Iwasaki M, Hanaoka T, Sasaki H, Tanaka T, Tsugane S. Urinary bisphenol-A concentration in infertile Japanese women and its association with endometriosis: a cross-sectional study. Environ Health Prev Med. 2007;12(6):258–64.
Silva MJ, Barr DB, Reidy JA, Malek NA, Hodge CC, Caudill SP, et al. Urinary levels of seven phthalate metabolites in the US population from the National Health and Nutrition Examination Survey (NHANES) 1999–2000. Environ Health Perspect. 2004;112(3):331–8.
Fujimoto T, Kubo K, Aou S. Prenatal exposure to bisphenol A impairs sexual differentiation of exploratory behavior and increases depression-like behavior in rats. Brain Res. 2006;1068(1):49–55.
Gupta C. Reproductive malformation of the male offspring following maternal exposure to estrogenic chemicals. Proc Soc Exp Biol Med. 2000;224(2):61–8.
Skakkebaek NE, Rajpert-De Meyts E, Main KM. Testicular dysgenesis syndrome: an increasingly common developmental disorder with environmental aspects. Hum Reprod. 2001;16(5):972–8.
Tsutsumi O. Assessment of human contamination of estrogenic endocrine-disrupting chemicals and their risk for human reproduction. J Steroid Biochem Mol Biol. 2005;93:325–30.
Nakai K, Suzuki K, Oka T, Murata K, Sakamoto M, Okamura K, et al. The Tohoku study of child development: a cohort study of effects of perinatal exposures to methylmercury and environmentally persistent organic pollutants on neurobehavioral development in Japanese children. Tohoku J Exp Med. 2004;202(3):227–37.
Ohshima Y. Do endocrine disruptors affect atopic predisposition. Allergology. 2004;18(5):387–92 (in Japanese).
NTP-CERHR. Expert panel report on the reproductive and developmental toxicity of bisphenol A. Research triangle park, NC: national toxicology program center for the evaluation of risks to human reproduction, 26 November 2007.
Takeuchi T, Tsutsumi O. Evaluation of bisphenol contamination in Humans. Obstet Gynecol Prac. 2003;52(13):2325–31 (in Japanese).
Völkel W, Colnot T, Csanady GA, Filser JG, Dekant W. Metabolism and kinetics of bisphenol A in humans at low doses following oral administration. Chem Res Toxicol. 2002;15(10):1281–7.
Kurebayashi H, Harada R, Stewart RK, Numata H, Ohno Y. Disposition of a low dose of bisphenol A in male and female cynomolgus monkeys. Toxicol Sci. 2002;68(1):32–42.
Pottenger LH, Domoradzki JY, Markham DA, Hansen SC, Cagen SZ, Waechter JM Jr. The relative bioavailability and metabolism of bisphenol A in rats is dependent upon the route of administration. Toxicol Sci. 2000;54(1):3–18.
Calafat AM, Kuklenyik Z, Reidy JA, Caudill SP, Ekong J, Needham LL. Urinary concentrations of bisphenol A and 4-nonylphenol in a human reference population. Environ Health Perspect. 2005;113(4):391–5.
Matsumoto A, Kunugita N, Kitagawa K, Isse T, Oyama T, Foureman GL, et al. Bisphenol A levels in human urine. Environ Health Perspect. 2003;111(1):101–4.
Tsukioka T, Terasawa J, Sato S, Hatayama Y, Makino T, Nakazawa H. Development of analytical method for determining trace amounts of BPA in urine samples and estimation of exposure to BPA. J Environ Chem. 2004;14(1):57–63.
Yang M, Kim SY, Chang SS, Lee IS, Kawamoto T. Urinary concentrations of bisphenol A in relation to biomarkers of sensitivity and effect and endocrine-related health effects. Environ Mol Mutagen. 2006;47(8):571–8.
Kodaira T, Kato I, Li J, Mochizuki T, Hoshino M, Usuki Y, et al. Novel ELISA for the measurement of immunoreactive bisphenol A. Biomed Res. 2000;21(2):117–21.
Inoue K, Wada M, Higuchi T, Oshio S, Umeda T, Yoshimura Y, et al. Application of liquid chromatography-mass spectrometry to the quantification of bisphenol A in human semen. J Chromatogr B Analyt Technol Biomed Life Sci. 2002;773(2):97–102.
Schwartz GJ, Feld LG, Langford DJ. A simple estimate of glomerular filtration rate in full-term infants during the first year of life. J Pediatr. 1984;104(6):849–54.
Arakawa C, Fujimaki K, Yoshinaga J, Imai H, Serizawa S, Shiraishi H. Daily urinary excretion of bisphenol A. Environ Health Prev Med. 2004;9:22–6.
Wolff MS, Teitelbaum SL, Windham G, Pinney SM, Britton JA, Chelimo C, et al. Pilot study of urinary biomarkers of phytoestrogens, phthalates, and phenols in girls. Environ Health Perspect. 2007;115(1):116–21.
Calafat AM, Ye X, Wong LY, Reidy JA, Needham LL. Exposure of the U.S. population to bisphenol A and 4-tertiary-octylphenol: 2003–2004. Environ Health Perspect. 2008;116(1):39–44.
Scientific Committee on Food (SCF). Opinion of the scientific committee on food on bisphenol A. European Commission Health and Consumer Protection Directorate-General, Directorate C-Scientific Opinions, C2-Management of scientific committees; scientific co-operation and networks, SCF/CS/PM/3936 Final, 3 May 2002.
Takai Y, Tsutsumi O, Ikezuki Y, Hiroi H, Osuga Y, Momoeda M, et al. Estrogen receptor-mediated effects of a xenoestrogen, bisphenol A, on preimplantation mouse embryos. Biochem Biophys Res Commun. 2000;270(3):918–21.
vom Saal FS, Hughes C. An extensive new literature concerning low-dose effects of bisphenol A shows the need for a new risk assessment. Environ Health Perspect. 2005;113(8):926–33.
Nakanishi J, Miyamoto K, Kawasaki H. Risk assessment of human health. In: New energy and industrial technology development organization and research center for chemical risk management, National Institute of Advanced Industrial Science and Technology, editors. Bisphenol A. Tokyo: Maruzen; 2005. pp. 55–117 (in Japanese).
Food sanitation law (relevant laws and regulations); 1994 (in Japanese).
The study circle for health and nutrition information, editor. The national nutrition survey in Japan, 2000 Ministry of Health, Labour and Welfare, Japan. Tokyo: Daiichi-Syuppan; 2002. p. 75 (in Japanese).
Yamaguchi K. Examination of renal function for newborn. Shusanki Igaku. 1976;6:392–8 (in Japanese).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yamano, Y., Miyakawa, S., Iizumi, K. et al. Long-term study of urinary bisphenol A in elementary school children. Environ Health Prev Med 13, 332–337 (2008). https://doi.org/10.1007/s12199-008-0049-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12199-008-0049-6