Abstract
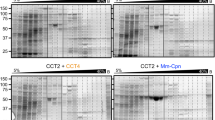
Studies on TCP1-1 ring complex (TRiC) chaperonin have shown its indispensable role in folding cytosolic proteins in eukaryotes. In a psychrophilic organism, extreme cold temperature creates a low-energy environment that potentially causes protein denaturation with loss of activity. We hypothesized that TRiC may undergo evolution in terms of its structural molecular adaptation in order to facilitate protein folding in low-energy environment. To test this hypothesis, we isolated G. antarctica TRiC (GaTRiC) and found that the expression of GaTRiC mRNA in G. antarctica was consistently expressed at all temperatures indicating their importance in cell regulation. Moreover, we showed GaTRiC has the ability of a chaperonin whereby denatured luciferase can be folded to the functional stage in its presence. Structurally, three categories of residue substitutions were found in α, β, and δ subunits: (i) bulky/polar side chains to alanine or valine, (ii) charged residues to alanine, and (iii) isoleucine to valine that would be expected to increase intramolecular flexibility within the GaTRiC. The residue substitutions observed in the built structures possibly affect the hydrophobic, hydrogen bonds, and ionic and aromatic interactions which lead to an increase in structural flexibility. Our structural and functional analysis explains some possible structural features which may contribute to cold adaptation of the psychrophilic TRiC folding chamber.









Similar content being viewed by others
References
Al-Fageeh MB, Smales CM (2006) Control and regulation of the cellular responses to cold shock: the responses in yeast and mammalian systems. Biochemical Journal (2):247–259. https://doi.org/10.1042/BJ20060166
Alimenti C, Ortenzi C, Carratore V, Luporini P (2003) Structural characterization of En-1, a cold-adapted protein pheromone isolated from the Antarctic ciliate Euplotes nobilii. Biochim Biophys Acta 1621:17–21. https://doi.org/10.1016/S0304-4165(03)00011-4
Bae E, Phillips GN Jr (2004) Structures and analysis of highly homologous psychrophilic, mesophilic, and thermophilic adenylate kinases. J Biol Chem 279(27):28202–28208. https://doi.org/10.1074/jbc.M401865200
Bergholz PW, Bakermans C, Tiedje JM (2009) Psychrobacter arcticus 273-4 uses resource efficiency and molecular motion adaptations for subzero temperature growth. J Bacteriol 191:2340–2352. https://doi.org/10.1128/JB.01377-08
Boo SY, Wong CMVL, Rodrigues KF, Najimudin N, Murad AMA, Mahadi NM (2013) Thermal stress responses in Antarctic yeast, Glaciozyma antarctica PI12, characterized by real-time quantitative PCR. Polar Biol 36:381–389. https://doi.org/10.1007/s00300-012-1268-2
Bukau B, Weissman J, Horwich A (2006) Molecular chaperones and protein quality control. Cell 125:443–451. https://doi.org/10.1016/j.cell.2006.04.014
Burley SK, Petsko GA (1986) Amino-aromatic interactions in proteins. FEBS Lett 203:139–143. https://doi.org/10.1016/0014-5793(86)80730-X
Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M, Mueller R, Nolan T, Pfaffl MW, Shipley GL, Vandesompele J, Wittwer CT (2009) The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem 55:4–622. https://doi.org/10.1373/clinchem.2008.112797
Campanaro S, Williams TJ, Burg DW, De Francisci D, Treu L, Lauro FM, Cavicchioli R (2011) Temperature-dependent global gene expression in the Antarctic archaeon Methanococcoides burtonii. Env Microb 13(8):2018–2038. https://doi.org/10.1111/j.1462-2920.2010.02367.x
Casanueva A, Tuffin M, Cary C, Cowan DA (2010) Molecular adaptations to psychrophily: the impacts of ‘omic’ technologies. Trends Microbiol 8:129–138. https://doi.org/10.1016/j.tim.2010.05.002
Criswell AR, Bae E, Stec B, Konisky J, Phillips GN Jr (2003) Structures of thermophilic and mesophilic adenylate kinases from the genus Methanococcus. J Mol Biol 330:1087–1099. https://doi.org/10.1016/S0022-2836(03)00655-7
Cuellar J, Yébenes H, Parker SK, Carranza G, Serna M, Valpuesta JM, Zabala JC, Detrich HW III (2014) Assisted protein folding at low temperature: evolutionary adaptation of the Antarctic fish chaperonin CCT and its client proteins. Biol Open 3(4):261–270. https://doi.org/10.1242/bio.20147427
Dekker C, Stirling PC, McCormack EA, Filmore H, Paul A, Brost RL, Costanzo M, Boone C, Leroux MR, Willison KR (2008) The interaction network of the chaperonin CCT. EMBO J 27:1827–1839. https://doi.org/10.1038/emboj.2008.108
Dougherty DA (1996) Cation-pi Interactions in Chemistry and Biology: A new view of benzene, phe, tyr, and trp. Science 271(5246):163–168. https://doi.org/10.1126/science.271.5246.163
Dougherty DA, Stauffer DA (1990) Acetylcholine binding by a synthetic receptor. Implications for biological recognition. Science 250(4987):1558–1560. PMID:2274786
Dunn AY, Melville MW, Frydman J (2001) Review: cellular substrates of the eukaryotic chaperonin TRiC/CCT. J Struct Biol 135:176–184. https://doi.org/10.1006/jsbi.2001.4380
Eisenberg D, Luthy R, Bowie JU (1997) Verify3D: assessment of protein models with three-dimensional profiles. Methods Enzymol 277:396–404. https://doi.org/10.1016/S0076-6879(97)77022-8
Elcock AH (1998) The stability of salt bridges at high temperatures: implications for hyperthermophilic proteins. J Mol Biol 284:489–502. PMID:9813132
Feldman DE, Thulasiraman V, Ferreyra RG, Frydman J (1999) Formation of the VHL-elongin BC tumor suppressor complex is mediated by the chaperonin TRiC. Mol Cell 4:1051–1061. https://doi.org/10.1016/S1097-2765(00)80233-6
Fields PA, Somero GN (1998) Hot spots in cold adaptation: localized increases in conformational flexibility in lactate dehydrogenase A4 orthologs of Antarctic notothenioid fishes. Proc Natl Acad Sci USA 95:11476–11481. https://doi.org/10.1073/pnas.95.19.11476
Firdaus-Raih M, Hashim NHF, Bharudin I, Abu Bakar MF, Huang KK, Alias H et al (2018) The Glaciozyma antarctica genome reveals an array of systems that provide sustained responses towards temperature variations in a persistently cold habitat. PLoS ONE 13(1):e0189947. https://doi.org/10.1371/journal.pone.0189947
Frydman J, Nimmesgern E, Ohtsuka K, Hartl FU (1994) Folding of nascent polypeptide chains in a high molecular mass assembly with molecular chaperones. Nature 370:111–117. https://doi.org/10.1038/370111a0
Fulda S, Gorman AM, Hori O & Samali A (2010) Cellular stress responses: cell survival and cell death. International Journal of Cell Biology, vol. 2010, 214074, 23. https://doi.org/10.1155/2010/214074
Gasteiger E, Hoogland C, Gattiker A, Duvaud S, Wilkins MR, Appel RD and Bairoch A (2005) Protein identification and analysis tools on the ExPASy server. In: John WM (ed) The proteomics protocols handbook, Humana press, pp 571-607
Goodchild A, Raftery M, Saunders NFW, Guilhaus M, Cavicchioli R (2004) Biology of the cold adapted archaeon, Methanococcus burtonii determined by proteomics using liquid chromatography-tandem mass spectrometry. J Proteome Res 3(6):1164–1176. https://doi.org/10.1021/pr0498988
Guenther MG, Yu J, Kao GD, Yen TJ, Lazar MA (2002) Assembly of the SMRT-histone deacetylase 3 repression complex requires the TCP-1 ring complex. Genes Dev 16:3130–3135. https://doi.org/10.1101/gad.1037502
Guex N, Peitsch MC (1997) SWISS-MODEL and the Swiss-PdbViewer: an environment for comparative protein modeling. Electrophoresis 18(15):2714–2723. https://doi.org/10.1002/elps.1150181505
Hartl FU (1996) Molecular chaperones in cellular protein folding. Nature 381:571–580. https://doi.org/10.1038/381571a0
Hartl FU, Hayer-Hartl M (2002) Molecular chaperones in the cytosol: from nascent chain to folded protein. Science 295:1852–1858. https://doi.org/10.1126/science.1068408
Hartl FU, Hayer-Hartl M (2009) Converging concepts of protein folding in vitro and in vivo. Nat Struct Mol Biol 16(6):574–581. https://doi.org/10.1038/nsmb.1591
Hartl FU, Bracher A, Hayer-Hartl M (2011) Molecular chaperones in protein folding and proteostasis. Nature 475:324–332. https://doi.org/10.1038/nature10317
Hashim N, Bharudin I, Nguong D, Higa S, Bakar F, Nathan S, Rabu A, Kawahara H, Illias R, Najimudin N, Mahadi N, Murad A (2013) Characterization of Afp1, an antifreeze protein from the psychrophilic yeast Glaciozyma antarctica PI12. Extremophiles 17:63–73. https://doi.org/10.1007/s00792-012-0494-4
Hensen SM, Heldens L, van Enckevort CM, van Genesen ST, Pruijn GJ, Lubsen NH (2013) Activation of the antioxidant response in methionine deprived human cells results in an HSF1-independent increase in HSPA1A mRNA levels. Biochimie 95:1245–1251. https://doi.org/10.1016/j.biochi.2013.01.017
Jolly C & Morimoto RI (2000) Role of the heat shock response and molecular chaperones in oncogenesis and cell Death. J Natl Cancer Inst. 92(19): 1564–1572. https://doi.org/10.1093/jnci/92.19.1564
Jones PG, Inouye M (1994) The cold-shock response-a hot topic. Mol Microbiol 11:811–818. https://doi.org/10.1111/j.1365-2958.1994.tb00359.x
Kalisman N, Adams CM, Levitt M (2012) Subunit order of eukaryotic TRiC/CCT chaperonin by cross-linking, mass spectrometry and combinatorial homology modeling. Proc Natl Acad Sci U S A 109:2884–2889. https://doi.org/10.1073/pnas.1119472109
Kalisman N, Schröder GF, Levitt M (2013) The crystal structures of the eukaryotic chaperonin CCT reveal its functional partitioning. Structure 21(4):540–549. https://doi.org/10.1016/j.str.2013.01.017
Kalman M, Ben-Tal N (2010) Quality assessment of protein model-structures using evolutionary conservation. Bioinformatics 26(10):1299–1307. https://doi.org/10.1093/bioinformatics/btq114
Kawamoto J, Kurihara T, Kitagawa M, Kato I, Esaki N (2007) Proteomic studies of an Antarctic cold-adapted bacterium, Shewanella livingstonensis Ac10, for global identification of cold-inducible proteins. Extremophiles 11:819–826. https://doi.org/10.1007/s00792-007-0098-6
Lanzarotti E, Biekofsky RR, Estrin DA, Marti MA, Turjanski AG (2011) Aromatic-aromatic interactions in proteins: beyond the dimer. J Chem Inf Model 51:1623–1633. https://doi.org/10.1021/ci200062e
Laskowski RA, MacArthur MW, Moss DS & Thornton JM (1993). PROCHECK: a program to check the stereochemical quality of protein structures. J Appl Crystallogr 26:283–291. https://doi.org/10.1107/S0021889892009944
Leitner A, Joachimiak LA, Bracher A, Mönkemeyer L, Walzthoeni T, Chen B, Pechmann S, Holmes S, Cong Y, Ma B (2012) The molecular architecture of the eukaryotic chaperonin TRiC/CCT. Struc 20:814–825. https://doi.org/10.1016/j.str.2012.03.007
Ma JC, Dougherty DA (1997) The Cation−π interaction. Chem Rev 97(5):1303–1324. https://doi.org/10.1021/cr9603744
Matthews BW (2001) Hydrophobic interactions in proteins. In: eLS. John Wiley & Sons Ltd, Chichester. https://doi.org/10.1038/npg.els.0002975
Melki R, Cowan NJ (1994) Facilitated folding of actins and tubulins occurs via a nucleotide- dependent interaction between cytoplasmic chaperonin and distinctive folding intermediates. Mol Cell Biol 14:2895-2904. PMCID: PMC358657
Melo F, Devos D, Depiereux E, Feytmans E (1997) ANOLEA: a www server to assess protein structures. Proc Int Conf Intell Syst Mol Bio 5:187–190. https://doi.org/10.1093/nar/gkh440
Melo F, Feytmans E (1998) Assessing protein structures with a non-local atomic interaction energy. J Mol Biol 277:1141–1152. https://doi.org/10.1006/jmbi.1998.1665
Morris AL, MacArthur MW, Hutchinson EG, Thornton JM (1992) Stereochemical quality of protein structure coordinates. Proteins Struct Funct Genet 12(4):345–364. https://doi.org/10.1002/prot.340120407
Phadtare S (2004) Recent developments in bacterial cold-shock response. Curr Issues Mol Biol 6:125–136
Phadtare S, Inouye M (2008) The cold shock response. EcoSal Plus 3. https://doi.org/10.1128/ecosalplus.5.4.2
Phadtare S, Alsina J, Inouye M (1999) Cold shock response and cold shock proteins. Curr Opin Microbiol 2(2):175–180. https://doi.org/10.1016/S1369-5274(99)80031-9
Perl D, Mueller U, Heinemann U, Schmid FX (2000) Two exposed amino acid residues confer thermostability on a cold shock protein. Nat Struct Bio 7:380–383. https://doi.org/10.1038/75151
Pettersen EF, Dgoddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Thomas EF (2004) UCSF Chimera-A visualization system for exploratory research and analysis. J Comput Chem 25(13):1605–1612. https://doi.org/10.1002/jcc.20084
Piette F, D’Amico S, Mazzucchelli G, Danchin A, Leprince P, Feller G (2011) Life in the cold: a proteomic study of cold-repressed proteins in the Antarctic bacterium Pseudoalteromonas haloplanktis TAC125. Appl Environ Microbiol 77(11):3881–3883. https://doi.org/10.1128/AEM.02757-10
Privalov PL (1990) Cold denaturation of proteins. Crit Rev Biochem Mol Biol 25(4):281–305. https://doi.org/10.3109/10409239009090612
Pucciarelli S, Marziale F, Di Giuseppe G, Barchetta S, Miceli C (2005) Ribosomal cold- adaptation: characterization of the genes encoding the acidic ribosomal P0 and P2 proteins from the Antarctic ciliate Euplotes focardii. Gene 360(2):103–110. https://doi.org/10.1016/j.gene.2005.06.007
Pucciarelli S, Parker SK, Detrich HW III, Melki R (2006) Characterization of the cytoplasmic chaperonin containing TCP-1 from the Antarctic fish Nototheinia coriiceps. Extremophiles 10(6):537–549. https://doi.org/10.1007/s00792-006-0528-x
Quevillon E, Silventoinen V, Pillai S, Harte N, Mulder N, Apweiler R, Lopez R (2005) InterProScan: protein domains identifier. Nucleic Acids Res 33(2):116–120. https://doi.org/10.1093/nar/gki442
Ramli A, Mahadi N, Shamsir M, Rabu A, Joyce-Tan K, Murad A, Illias R (2012) Structural prediction of a novel chitinase from the psychrophilic Glaciozyma antarctica PI12 and an analysis of its structural properties and function. J Comput Aided Mol Des 26(8):947–961. https://doi.org/10.1007/s10822-012-9585-7
Ringer AL, Senenko A, Sherrill CD (2007) Models of S/π interactions in protein structures: comparison of the H2S–benzene complex with PDB data. Prot Soc 16(10):2216–2223. https://doi.org/10.1110/ps.073002307
Schwede T, Kopp J, Guex N, Peitsch MC (2003) SWISS-MODEL: an automated protein homology-modeling server. Nucleic Acids Res 3113:3381–3385. https://doi.org/10.1093/nar/gkg520
Slutsky MM, Marsh ENG (2004) Cation-π interactions studied in a model coiled-coil peptide. Protein Sci 13(8):2244–2251. https://doi.org/10.1110/ps.04702104
Somero GN (2004) Adaptation of enzymes to temperature: searching for basic “strategies”. Comp Biochem Physiol 139B:321–333. https://doi.org/10.1016/j.cbpc.2004.05.003
Sonnhammer EL, Eddy SR, Durbin R (1997) Pfam: a comprehensive database of protein domain families based on seed alignments. Proteins 28(3):405–420. https://doi.org/10.1093/nar/30.1.276
Spiess C, Meyer AS, Reissmann S, Frydman J (2004) Mechanism of the eukaryotic chaperonin: protein folding in the chamber of secrets. Trends Cell Biol 14(11):598–604. https://doi.org/10.1016/j.tcb.2004.09.015
Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22(22):4673–4680. https://doi.org/10.1093/nar/22.22.4673
Thulasiraman V, Yang CF, Frydman J (1999) In vivo newly translated polypeptides are sequestered in a protected folding environment. EMBO Jour 18:85–95. https://doi.org/10.1093/emboj/18.1.85
Tomoyasu T, Mogk A, Langen H, Goloubino P, Bukau B (2001) Genetic dissection of the roles of chaperones and proteases in protein folding and degradation in the Escherichia coli cytosol. Mol Microbiol 40:397–413. https://doi.org/10.1046/j.1365-2958.2001.02383.x
Tsai C, Aslam K, Drendel HM, Asiago JM, Goode KM, Paul LN, Rochet JC, Hazbun TR (2015) Hsp31 is a stress response chaperone that intervenes in the protein Misfolding process. J Biol Chem 290(41):24816–24834. https://doi.org/10.1074/jbc.M115.678367
Valpuesta JM, Martín-Benito J, Gómez-Puertas P, Carrascosa JL, Willison KR (2002) Structure and function of a protein folding machine: the eukaryotic cytosolic chaperonin CCT. FEBS Lett 529:11–16. https://doi.org/10.1016/S0014-5793(02)03180-0
Vieille C, Zeikus GJ (2001) Hyperthermophilic enzymes: sources, uses, and molecular mechanisms for Thermostability. Microbiol Mol Biol Rev 65(1):143–143. https://doi.org/10.1128/MMBR.65.1.1-43.2001
Yam AY, Xia Y, Lin HT, Burlingame A, Gerstein M, Frydman J (2008) Defining the TRiC/CCT interactome links chaperonin function to stabilization of newly made proteins with complex topologies. Nat Struct & Mol Biol 15:1255–1262. https://doi.org/10.1038/nsmb.1515
Yébenes H, Mesa P, Muñoz IG, Montoya G, Valpuesta JM (2011) Chaperonins: two rings for folding. Trends Biochem Sci 36:424–432. https://doi.org/10.1016/j.tibs.2011.05.003
Yusof NA, Abu Bakar FD, Mahadi NM, Murad AMA (2017) Comparative modeling of TCP1 ring complex (TRiC) from a psychrophilic yeast, Glaciozyma antarctica. Transactions on Science and Technology 4(3–3):324–329 ISSN 2289-8786
Yusof NA, Abu Bakar FD, Illias RM, Mahadi NM, Murad AMA (2015) In silico characterisation of the Glaciozyma antarctica genome: mining the molecular chaperones. Malaysian Applied Biology 44(1):161–165 ISSN 0126-8643
Zacharias N, Dougherty DA (2002) Cation–π interactions in ligand recognition and catalysis. Trends Pharmacol Sci 23(6):281–287. https://doi.org/10.1016/s0165-6147(02)02027-8
Zauhar RJ, Colbert CL, Morgan RS, Welsh WJ (2000) Evidence for a strong sulfur–aromatic interaction derived from crystallographic data. Biopolymers 53:233–248. https://doi.org/10.1002/(SICI)1097-0282(200003)53:3<233::AID-BIP3>3.0.CO;2-4
Zheng S, Ponder MA, Shih JY, Tiedje JM, Thomashow MF, Lubman DM (2007) A proteomic analysis of Psychrobacter articus 273-4 adaptation to low temperature and salinity using a 2-D liquid mapping approach. Electrophoresis 28:467–488. https://doi.org/10.1002/elps.200600173
Zhuravleva A, Radford SE (2014) How TRiC folds tricky proteins. Cell 159(6):1251–1252. https://doi.org/10.1016/j.cell.2014.11.029
Acknowledgments
We are grateful to the Ministry of Science, Technology and Innovation, Malaysia, (MOSTI) for funding our project under grant number 02-05-20-SF0007. We thank Prof Jamie Rossjohn for the opportunity to use his lab at Monash University and synchrotron facilities in Australia. Special thanks to Dr. Travis Beddoe from La Trobe University who assisted us with his advice and technical guidance. We are thankful to the Ministry of Higher Education Malaysia for the FRG0463-2017 grant for funding the continuous culturing of Glaciozyma antarctica in Biotechnology Research Institute, Universiti Malaysia Sabah. We also thank those who were involved in the structural project of Glaciozyma antarctica in Malaysia Genome Institute and others who assisted with technical assistance and helpful discussions.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOC 5343 kb)
Rights and permissions
About this article
Cite this article
Yusof, N.A., Kamaruddin, S., Abu Bakar, F.D. et al. Structural and functional insights into TRiC chaperonin from a psychrophilic yeast, Glaciozyma antarctica. Cell Stress and Chaperones 24, 351–368 (2019). https://doi.org/10.1007/s12192-019-00969-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12192-019-00969-1