Abstract
Background
Posttraumatic stress disorder (PTSD) is a significant risk factor for cardiovascular and metabolic disease.
Purpose
The purpose of the current review is to evaluate the evidence suggesting that PTSD increases cardiovascular and metabolic risk factors, and to identify possible biomarkers and psychosocial characteristics and behavioral variables that are associated with these outcomes.
Methods
A systematic literature search in the period of 2002–2009 for PTSD, cardiovascular disease, and metabolic disease was conducted.
Results
The literature search yielded 78 studies on PTSD and cardiovascular/metabolic disease and biomarkers.
Conclusions
Although the available literature suggests an association of PTSD with cardiovascular disease and biomarkers, further research must consider potential confounds, incorporate longitudinal designs, and conduct careful PTSD assessments in diverse samples to address gaps in the research literature. Research on metabolic disease and biomarkers suggests an association with PTSD, but has not progressed as far as the cardiovascular research.
Similar content being viewed by others
Introduction
The past decade of research has provided evidence that individuals with posttraumatic stress disorder (PTSD) report more health complaints, suffer from more physician-diagnosed medical conditions [1], and exhibit higher health care utilization [2]. However, further evidence is needed to determine whether PTSD increases risk of physical illness. The present review focuses on research on other biomarkers and physiological mechanisms of the association between PTSD and physical illness. In particular, the review details results on two classes of disease commonly associated with PTSD: cardiovascular and metabolic disease. Despite treatment efforts, PTSD remains prevalent, and it is important to monitor the physical health implications of PTSD and inform prevention and treatment efforts. Because previous reviews of PTSD and cardiovascular disease [3] and hostility [4] have included comprehensive characterizations of the pre-2002 literature, we chose to update this literature by focusing our search on publications between 2002 and 2009. A number of recent reports have contributed to this quickly growing literature, and review of recent findings is needed to identify important research and clinical targets.
In this review, we propose a model of the relationship between PTSD and cardiovascular and metabolic disease. Although research has linked PTSD to a number of health problems, there has been a lack of specificity in terms of which disease processes may be most related to PTSD. Because different diseases may be related to PTSD through different mechanisms or pathways, this review focuses on cardiovascular and metabolic disease specifically. In addition, a previous review by Boscarino [5] detailed findings and mechanisms supporting the possibility that PTSD could alter disease outcomes through disrupted hypothalamic-pituitary-adrenal axis (HPA) and sympathoadrenomedullary (SAM) functioning, so this review focuses on cardiovascular and metabolic mechanisms. Because individuals with PTSD have been shown to exhibit higher levels of anger and hostility [6], we explore the role of mediators (e.g., hostility) of the relationship between PTSD and cardiovascular/metabolic disease. We also discuss the potential roles of moderators in cardiovascular/metabolic outcomes.
Identification of Studies for Review
Articles examining PTSD, cardiovascular disease (CVD), and metabolic disease were identified by searching the Published International Literature on Traumatic Stress, MEDLINE, and Psychological Information databases for articles published between January 2002 and April 2009. Articles were included if they met the following criteria: (1) inclusion of at least one measure of cardiovascular- or metabolic-related disease, mortality, or biomarkers; (2) original data or a new analysis of previously published data, rather than a review of the literature; (3) focused on the development of disease or biomarkers in those with PTSD; and (4) availability in English language. Animal studies were excluded. Search terms included the following: PTSD, cardio*, diabet*, metabol*, heart rate, and blood pressure. Studies focusing on the development of PTSD in response to medical trauma (e.g., PTSD developed with myocardial infarction as the index trauma or prevalence of PTSD in patients with diabetes), the prediction of PTSD by peritraumatic physical symptoms, or physiological response to treatment were not included in the primary literature search. Search terms were selected to focus on the diseases and biomarkers of primary interest and restricted this review from other worthy topics such as infectious disease. In addition to the examination of electronic databases, the references of all retrieved articles were reviewed to identify additional relevant investigations. These procedures yielded a total of 78 published reports. In addition to these articles, two studies were included because they were known to be in press and a number of references were included to provide historical or scientific context, as well as to review other critical variables in this field of research, including depression, behavioral health, and hostility.
Model of Posttraumatic Stress Disorder, Cardiovascular Disease, and Metabolic Disease
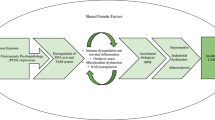
A substantial literature documents a potential relationship between PTSD and increased cardiovascular reactivity to trauma-related cues, disturbed sleep physiology, autonomic hyperarousal, and altered HPA activity [7]. A possible model to evaluate the relationships between PTSD and health is presented in Fig. 1. Originally presented by Schnurr and Jankowski [8], this model proposes that certain individuals are more likely to endure traumatic stress and that both traumatic stress and PTSD are directly related to several physiological reactivity indices and disease biomarkers. In addition, the model conceptualizes PTSD as being indirectly related to reactivity and biomarkers through psychological states such as hostility and health risk behaviors commonly associated with PTSD. Finally, the model proposes several pre-clinical disease variables that convey the effects of physiological reactivity and disease biomarkers to medical morbidity and mortality. Given the differential prevalence rates in PTSD by gender, there may also be important gender differences in the trajectory of the disorder. However, because research in this area is lacking, gender is not proposed as a moderator in this model.
Model of the influences of traumatic stress risk, PTSD, psychological states, and health behavior on pre-clinical disease markers. It is proposed that pre-clinical disease biomarkers then progress to cardiovascular/metabolic disease through autonomic dysregulation, endothelial dysfunction, metabolic syndrome, and insulin resistance. This figure is modified from Schnurr & Jankowski [8]
Cardiovascular and Metabolic Morbidity/Mortality in PTSD
PTSD and Morbidity and Mortality of Cardiovascular Disease
Initial evidence of a link between PTSD and CVD was drawn from investigations of CVD following traumatic stress. A longitudinal study of traumatic stress resulting from prolonged war in Beirut found that individuals with more war-related traumatic experiences were at greater risk for CVD-related mortality. The relationship between trauma and CVD differed by gender such that CVD-related mortality was more evident in women who suffered personal traumas, such as injuries or family deaths, while it was more prominent in men who had suffered property loss, work-related problems, or displacement from home [9]. In a study of cardiovascular morbidity in World War II prisoners of war, increased risk of CVD was present in those who developed PTSD [10]. A recent review summarizing the results of seven studies published between 1999 and 2006 found partial support for an association between PTSD and cardiovascular and metabolic disease [3]. PTSD was significantly associated with medical morbidity in four out of the six included studies on circulatory disorders [10–13]. A study of Vietnam veterans found that those veterans with PTSD exhibited elevated rates of CVD relative to combat-exposed veterans without PTSD [12]. In a review of medical records of male veterans who completed self-report measures of PTSD symptoms in 1990 and did not have coronary heart disease at the time of the baseline assessment, PTSD symptoms were associated with increased risk of experiencing either nonfatal myocardial infarction or onset of fatal coronary heart disease over an 11-year period [14]. A telephone-interview-based 30-year follow-up study with Vietnam era veterans noted that those veterans at PTSD were at increased risk of CVD-related mortality [15]. In a study of Australian veterans, PTSD was associated with increased risk of hypertension [16]. This study was unique and significant due to its use of the Structured Clinical Interview for the Diagnostic and Statistical Manual for PTSD diagnosis, interview assessment of medical disorders, and adjustment for demographic covariates, combat exposure, and peritraumatic dissociation.
While much of the research on medical morbidity has occurred in male veterans, there is preliminary evidence that those findings are present in other populations. A study of civilian survivors of a man-made disaster reported that vascular disorders were more common in those who developed PTSD [17]. In addition, data from the National Comorbidity Survey of United States civilians support an increased risk of hypertension in PTSD [18], a finding that was also evident in PTSD-diagnosed family members of soldiers killed in the wars in Bosnia and Herzegovina [19]. An association between PTSD and CVD was also observed in firefighters [20] and Native American Indians [21]. Prospective epidemiologic evidence from the Baltimore cohort of the Epidemiologic Catchment Area Survey indicates that this relationship might generalize to civilian women, as the presence of at least five PTSD symptoms was related to increased 14-year incidence of coronary heart disease in women [22].
Concordant with the evidence of a possible relationship between PTSD and CVD, available research supports a link to both CVD-related and all-cause mortality. Data analysis from a random sample of 4,462 Vietnam era veterans suggested that the predictive power of PTSD for mortality may be comparable to that of common disease indicators such as inflammation [23]. In fact, in models statistically controlling for demographic and military variables, behavioral health variables, substance abuse, depression, and baseline disease pathology measures, PTSD was associated with an approximate twofold increase in both all-cause and cardiovascular-related mortality [24]. Recent evidence suggests that subtypes of PTSD may be particularly associated with morbidity and mortality [25]. Subtypes of externalizing and internalizing have been identified in previous studies [7], and these subtypes may be significantly related to mortality compared to individuals with a low pathology subtype [25]. Investigations of PTSD and physical morbidity suggest an association between the two, but there is a clear need for epidemiologic research using longitudinal, prospective methods and investigation of potential moderating variables. Unfortunately, the examination of gender differences in the cardiovascular physiology of PTSD is also relatively understudied.
PTSD and Morbidity of Type 2 Diabetes
Although the research is not as extensive as that on CVD, there is evidence that PTSD is associated with the occurrence of Type 2 diabetes. A recent review found partial evidence for the relationship: of the four studies that included diabetes as an outcome, positive associations with PTSD were observed in two [3, 11, 26]. After controlling for a range of covariates, including age, education, race, intelligence, income, geographic region, Army volunteer status, number of times married, and history of antisocial personality, alcohol abuse, drug abuse, and history of cigarette smoking, PTSD was associated with an adjusted odds ratio of 2.9 for insulin-dependent diabetes mellitus in veterans [5]. In men sampled from a primary care setting, trauma history was associated with diabetes, but PTSD was not [27]. PTSD was found to be related to increased risk of diabetes in a large primary care sample [26]. Medical eligibility data demonstrated a positive link in female children and adolescents between PTSD and problems in a combined category of endocrine, metabolic, and immune function [28]. The association between self-reported diabetes and PTSD appears to generalize at least somewhat to community population adults, although a large survey of Canadian civilians found no association between PTSD and diabetes after adjusting for demographic and psychiatric variables [11, 29, 30]. Unfortunately, there are no studies to date that longitudinally examine specific biomarkers of CVD and type 2 diabetes risk in individuals with PTSD. In addition, possible gender differences in PTSD and diabetes in adults have not yet been evaluated.
Biomarkers of Cardiovascular and Metabolic Disease in PTSD
Autonomic Dysregulation
The autonomic nervous system regulates vital body functions such as heart rate, respiration rate, digestion, and salivation. It includes the sympathetic nervous system (SNS), which promotes short-term adaptation to immediate stressors through the fight-or-flight response and the parasympathetic nervous system (PNS), which reduces heart and respiration rates in order to conserve and restore energy. Autonomic dysregulation can arise from disruption of either the sympathetic or parasympathetic nervous systems, or disruption of the interplay between these two systems. Not surprisingly, recent research examining both SNS and PNS suggests they combine to produce the autonomic dysregulation associated with PTSD [31]. Consequently, measurement and consideration of both the SNS and the PNS are critical to developing a complete understanding of PTSD and autonomic dysregulation. In addition, early research on autonomic dysregulation in PTSD compared Lebanon war veterans with PTSD to those without, noting that veterans with PTSD reported more cardiovascular symptoms and low effort tolerances during a cardiovascular task [32]. More extensive alteration of cardiovascular indicators has been observed in individuals with chronic PTSD [33].
Heart Rate
Although a few studies have not detected an association between trauma cues and heart rate for individuals with PTSD (compared to trauma-exposed individuals with no PTSD) [34, 35], most research has found that individuals with PTSD exhibit a more pronounced increase in heart rate in response to trauma cues [36–39]. This reactivity may be relevant to increased risk of cardiovascular disease, as elevated resting heart rate has been shown to be predictive of earlier mortality from CVD [40]. It should be noted that there is some intragroup variability in the relationship between PTSD and increased heart rate response to trauma cues: 23% of a sample of motor vehicle accident survivors with PTSD, for example, did not demonstrate a significant increase in heart rate when listening to an audio description of the accident [41]. A number of studies have also observed increased heart rate in people with PTSD when no traumatic stimulus is being presented [36, 42]. Meta-analyses of laboratory studies have found that both men and women with PTSD show increased basal heart rate and blood pressure compared to both trauma- and non-trauma-exposed individuals without PTSD [33]. A study of comparative laboratory assessments showed that civilians with PTSD had higher basal heart rates than their non-PTSD counterparts, while police officers with PTSD did not [43]. Buckley and colleagues [44] found that increased basal heart rates were independent of the contributions of behavioral health variables such as body mass index (BMI) and smoking. Laboratory assessments of heart rate have been complemented by clinical assessments. Retrospective chart reviews of female veterans found that although there were no group differences in blood pressure or BMI, those with PTSD had elevated resting heart rates [45]. Elevated basal heart rate in PTSD has also been evident in studies using ambulatory monitoring [44, 46–48].
While elevated heart rate in PTSD is generally interpreted as being a result of the disorder, it is possible that hyperarousal of the cardiovascular system promotes heightened vulnerability to the development of PTSD. This hypothesis was investigated in a monozygotic twins study in which only one of each pair had been exposed to combat in the Vietnam War. Approximately half of the Vietnam veterans had developed PTSD, resulting in a sample consisting of combat veterans with and without PTSD as well as people who had not been exposed to combat. Heart rate responses to loud noises were elevated in combat veterans with PTSD, but not in their twins or the combat-exposed non-PTSD group [49], which suggests that this physiological reactivity was influenced by the development of PTSD.
Research on stressor stimuli that are not trauma-specific has yielded conflicting results. This contrast was evident in a study in which exaggerated heart rate reactivity to trauma cues was not mirrored by a larger increase in PTSD participant reactions to non-trauma negative cues in a sample of motor vehicle accident survivors [36]. A study of women who had survived assault found that those who developed PTSD did not exhibit an elevated heart rate response to a loud noise 1 month after the assault, but did 6 months after the assault [50]. Recent research in a sample of both men and women with various traumas found that both acute and chronic PTSD associated with an elevated heart rate response to trauma cues and an exaggerated startle response to loud noises [51]. While research to date indicates that people with PTSD have increased cardiovascular reactivity to trauma cues, the physiological response to general environmental stressors appears to be either similar or diminished relative to those without PTSD. When confronted with a cognitive challenge, a group of patients with PTSD responded with significantly higher cortisol, but similar heart rate and blood pressure compared to healthy controls [52]. In a study of general environmental stressors, participants with PTSD exhibited less physiological responsiveness to demands of laboratory tasks, relative to traumatized participants with no PTSD. When a response inhibition cue was added to the task, participants without PTSD exhibited the expected increases in SNS activity as indicated by skin conductance, but those with PTSD had smaller increases or no increase at all. Finally, while traumatized people without PTSD had increases in both heart rate and skin conductance when they could receive a monetary reward for performance on a lab task, those with PTSD exhibited a blunted physiological response [53]. Further evidence of physiological hyporeactivity to stressors was observed in a study of primarily female, non-veteran participants that compared people with PTSD to those with other anxiety disorders. Relative to other anxiety disorders, PTSD was associated with reduced eyeblink startle and heart rate increases to sentences designed to elicit physiological fear responses [54]. In a study of male and female Gulf War veterans with chronic fatigue, those with comorbid PTSD failed to exhibit the expected increase in blood pressure in response to speaking and mental arithmetic tasks requiring mental effort [55]. These results contradict observations suggesting general hyperarousal in people with PTSD and raise the possibility that physiological changes associated with PTSD could impair physiological responsiveness to non-trauma-related environmental stressors. It is possible that the characteristics of the stressor explain some of the inconsistencies in physiological responsiveness in PTSD. Nevertheless, given the conflicting nature of these results, further research is needed to establish that the physiological reactions noted in previous studies can be replicated and that the reactions are persistent across time in longitudinal studies.
In addition to the cardiovascular startle response, the time it takes for the cardiovascular system to recover to the baseline level following startle has also been used as a measure of dysfunction in PTSD. In a study using this methodology in a sample of combat veterans in treatment for PTSD, the severity of PTSD symptoms was related to delayed heart rate recovery following startle [56]. Although most of this research has been conducted in men, recent years have seen some research on the heart rate response to startle in women. In a study of women who served as nurses in Vietnam, those with current PTSD exhibited more dramatic startle responses, as measured by heart rate, than participants with either past or no history of PTSD [57]. However, skin conductance responses were not significantly larger in the current PTSD group. Because heart rate response is determined by both SNS and PNS components and the skin conductance response is primarily an indicator of SNS activity, the authors interpreted this discrepancy as an indication that the increased heart rate responses were due to decreased parasympathetic tone [57]. The autonomic trauma cue reactivity frequently observed in people with PTSD may be complicated by second-order conditioning of trauma cues. Due to repeated fear responses, cues related to the index trauma could function as unconditioned stimuli. However, evidence from heart rate conditioning paradigms has been inconsistent with regard to heart rate conditioning to non-trauma aversive stimuli [58, 59].
Blood Pressure
Although the effects are not as large or as consistent, findings of elevated blood pressure in PTSD have generally mirrored existing research on heart rate [33, 60]. Relative to traumatized individuals without PTSD, police officers with PTSD exhibited exaggerated blood pressure increases in response to trauma scripts [43]. In a study of eight male Vietnam veterans with combat-related PTSD, viewing videotapes of combat scenes induced negative mood and higher anxiety and was associated with increases in heart rate as well as both systolic and diastolic blood pressure [61]. A more dramatic blood pressure increase during affective distress has also been observed in studies monitoring ambulatory blood pressure [44]. Data from a small (n = 11) sample of veterans with PTSD indicated no significant relationship between norepinephrine and blood pressure, which suggests that irregular blood pressure findings in PTSD may be due in part to an absence of regulation by norepinephrine [62]. In a study comparing participants with PTSD to those with borderline personality disorder who had suffered childhood traumatic events, PTSD was related to significantly greater systolic, but not diastolic, blood press response to trauma scripts [63]. That there was no between-group difference in blood pressure response to neutral or abandonment scripts affirms a stronger cardiovascular response to trauma cues relative to the response to general stressors.
Meta-analysis has also suggested a trend toward higher basal blood pressure in PTSD [33]. Research on a sample of people with PTSD replicated previous observations of elevated baseline systolic and diastolic blood pressure [64]. Furthermore, these participants exhibited significantly greater increases in blood pressure following the administration of corticotrophin-releasing hormone [64].
Endothelial Dysfunction
Preliminary research has estimated the influence of PTSD on CVD by assessing endothelial dysfunction. Early research on medical morbidity from the Veterans Affairs Normative Aging Study suggested that self-reported PTSD symptoms were associated with increased risk of onset of arterial disorders [65]. Because endothelial dysfunction is recognized as the initial step in the atherosclerotic process and its progression [66, 67], assessment of endothelial functioning offers a potential pre-clinical marker of the development of arterial disorders as well as an additional method to utilize in research on CVD in PTSD. Endothelial dysfunction can be measured using flow-mediated dilation (FMD), which uses ultrasound images to follow changes in vessel diameter in response to increased blood flow. In arteries lined by healthy endothelium, increased flow causes dilatation of the vessel, but this response is compromised by endothelial dysfunction. FMD reveals the presence of endothelial dysfunction in individuals at high risk of atherosclerosis prior to the clinical manifestation of vascular disease [68, 69].
In a mixed sample of male and female police officers who completed the Impact of Event scale as a measure of PTSD symptomatology, FMD and carotid artery thickness were used to assess endothelial function. Compared to police officers with the lowest PTSD symptomatology, those in the severe range exhibited approximately half the brachial reactivity [70]. In contrast, carotid artery intima-media thickness did not differ by PTSD symptomatology. This study provides evidence that PTSD symptoms are related to pre-clinical markers of disease, but the study is limited due to the use of self-report measures rather than a clinician symptom rating sufficient for establishing a PTSD diagnosis.
Endothelial dysfunction can also be assessed by measuring circulating proteins that promote blood coagulation. In a small study comparing physically healthy patients with PTSD to traumatized controls, those with PTSD had higher levels of soluble tissue factor, although this effect was not independent of emotional distress and was not observed in two other endothelium-derived circulating proteins [71]. Another analysis of these data found some evidence for an association between an increase in blood clotting factors and PTSD symptom severity, although not PTSD diagnosis [72].
Baroreceptor Sensitivity
Mediated by central baroreceptor feedback loops, blood pressure variability is regulated by vagal control of heart rate and the direct effects of sympathetic activity on vasculature [73]. Individuals with reduced cardiac autonomic control, as indicated by reduced heart rate variability (HRV), have greater blood pressure variability due to a diminished buffering capacity [73, 74]. Baroreceptors are pressure-sensing nerves that regulate parasympathetic activity. Consequently, baroreceptor sensitivity has been used as a measure of PNS tone. Prominence of the PNS in cardiovascular symptoms in PTSD is derived from several studies in controlled laboratory conditions that have demonstrated a relationship between PTSD and reduced PNS control of the heart [75–77]. Parasympathetic components of cardiovascular functioning could also be influenced by the immediate environment, as illustrated by a study in which electrocardiogram assessments of children with PTSD were recorded while they interacted with parents [78].
An investigation of smokers with PTSD found that while baroreceptor sensitivity was not altered in men, it was significantly decreased in women [77]. Subsequent research extended these findings to a mixed sample of female smokers and non-smokers with PTSD in observing decreased baroreceptor sensitivity at rest. Furthermore, while baroreceptor sensitivity decreased for all participants during an anger recall task, the change was less dramatic for women with PTSD, possibly due to their lower resting values [76]. More recently, in a study of women with and without PTSD, an interaction was detected between baroreceptor sensitivity and objective sleep parameters [79]. Lower baroreceptor sensitivity was associated with poorer sleep in women with PTSD, but not in those without PTSD [79].
Parasympathetic Nervous System Measures
Because it is heavily influenced by the autonomic nervous system and involves both the SNS and PNS in its functioning, heart rate variability has often been used as an index of autonomic function. Heart rate variability is widely recognized as an important index of autonomic function that is useful as a prognostic indicator of risk for both CVD and metabolic syndrome. Previous research has indicated that reduced 24-h HRV independently predicts mortality in patients with stable CVD [80] or a recent myocardial infarction [81]. More recent evidence also supports the prognostic significance of HRV as a predictor of mortality in patients with heart failure [82]. A number of previous studies have reported reduced levels of short-term HRV in participants with anxiety disorders as well as in those with PTSD [83]. While these findings suggest that impaired HRV may contribute to risk in PTSD patients, a deeper understanding of the role of the autonomic nervous system in the increased risk of mortality in PTSD would be aided by the use of 24-h electrocardiogram (ECG) recordings, a method that can offer an improved estimation of risk over short-term ECG recordings [84]. Use of 24-h ECG recordings may be especially relevant to individuals with PTSD, as their reduced sleep duration and sleep efficiency may lead to reduced levels of ambulatory HRV [85]. Daily stressors and worry have also been associated with low HRV, during both wake and sleep [85]. Sleep and wake have profoundly different effects on HRV, with parasympathetic control of heart rate being substantially higher during sleep periods [86, 87]. Preliminary data suggest that circadian variation in HRV may be impaired in some veterans post-deployment, a group at high risk for PTSD [88]. These findings suggest that people with PTSD have alterations in HRV that would be better characterized by ecologically valid assessments that include overnight measurement of HRV.
The influence of the PNS on autonomic dysfunction has been assessed using respiratory sinus arrhythmia (RSA), the changes in heart rate rhythm associated with inspiration and expiration [83]. RSA reflects vagal tone and is one index of PNS function. One study noted that the elevated basal heart rate frequently observed in PTSD was only present in those with lower respiratory sinus arrhythmia, an indication of diminished PNS activity [83]. Further evidence of the prominent role played by the PNS in autonomic dysfunction in PTSD comes from a study that observed significantly lower RSA in participants with PTSD listening to trauma scripts than in those listening to neutral scripts, which suggests stress-induced suppression of the PNS. The heart rate increase associated with the trauma script for all participants was of longer duration in those participants with lowered RSA [89].
Cardiovascular functioning can also be assessed using power spectral analysis. The total heart variance is assessed with various outcome measures, including high frequency, which indicates vagal activity from the PNS; and low frequency, which indicates both SNS and PNS activity; and very low frequency [90]. Increased risk of CVD in PTSD could be influenced by elevated noradrenergic responsiveness. This interpretation is supported by similarities between the hyperarousal symptoms of PTSD and effects observed immediately following administration of catecholamines such as epinephrine and norepinephrine [90].
In a study comparing people with PTSD to those with panic disorder and to healthy controls, PTSD was associated with increased baseline sympathetic nervous system activation, as measured by the electrodermal activity and heart rate [31]. PTSD was also associated with diminished parasympathetic control of heart rate as measured by RSA. When participants were anticipating electric shocks, those with PTSD exhibited diminished electrodermal sensitivity relative to the other groups. These results support previous interpretations of autonomic dysregulation in PTSD that emphasized the interplay of sympathetic and parasympathetic components [78].
Although most evidence suggests that autonomic dysfunction is the result, rather than the cause, of chronic disease, some studies have documented autonomic dysfunction (as measured by RSA) among at risk individuals prior to disease onset [91]. Impaired autonomic control of heart rate is an early event in the progression of type 2 diabetes and is often present at the time of diagnosis [92–94]. Autonomic dysfunction is also present in children newly diagnosed with insulin-dependent diabetes mellitus [95]. These results raise the possibility that decreased vagal tone may play a role in the pathogenesis of certain chronic diseases, including CVD and type 2 diabetes. Recent research has provided further evidence of the role of autonomic dysfunction in the onset of chronic disease. In overnight fasting healthy volunteers, reduced autonomic function (as measured by baroreceptor sensitivity) was significantly associated with fasting plasma insulin after controlling for other predictors of baroreceptor sensitivity, such as age, blood pressure, and BMI [96]. HRV parameters were lower in diabetic patients with chronic complications compared to those without chronic complications [97].
Lipids
A number of studies provide evidence that PTSD is linked to CVD, but comparatively fewer studies have investigated the relationship between PTSD and metabolic abnormalities. However, some research on PTSD and cardiovascular/metabolic disease has included investigation of elevated lipids. In a study comparing people with PTSD to people with major depressive disorder (MDD), PTSD was associated with a number of lipid variables, including elevated cholesterol, low-density lipoproteins, and triglycerides, as well as decreased high-density lipoproteins [98]. A large medical record review of veterans enrolled in primary care split patients into the following four groups: PTSD only; MDD only; PTSD with comorbid MDD; and a comparison group with neither disorder. Using these groups, the authors found that among patients with depression, those with comorbid PTSD were 56% more likely to have cholesterol above 135 mg/dL and 38% more likely to have a low-density lipoprotein level above 110 mg/dL [99]. The group with comorbid PTSD and depression also had significantly higher weight and BMI. However, effect sizes were generally small for either PTSD or depression alone. Karlovic and colleagues [100] conducted a similar study with male Croatian combat veterans. They split participants into PTSD, MDD, and PTSD comorbid with MDD groups to compare to a healthy male control group, comprising mostly hospital employees. In this study, veterans with PTSD had significantly higher cholesterol, low-density lipoproteins, low-density/high-density lipoprotein ratio, and triglyceride concentrations relative to not only healthy controls, but also to veterans with MDD. Veterans with comorbid PTSD and MDD had elevations similar to those seen in the PTSD only group [100]. High-density lipoproteins were lowest in both groups comprising people with PTSD [100]. An additional study of Croatian soldiers with PTSD also found elevated lipids and cholesterol values relative to a healthy control group [101]. A study of police officers found that, relative to officers without PTSD, those with PTSD had elevated total cholesterol, triglycerides, and low-density lipoproteins. The effects on total cholesterol and triglyceride elevations were independent of socio-demographics, BMI, and tobacco, alcohol, and medication use [102].
Allostatic Load Model
Friedman and McEwen [103] provided a comprehensive theory for understanding links between PTSD and physical illness by using the allostasis model. Allostasis refers to the process of adaptation to stressors to achieve stability through change [104]. While short-term adaptation to stress is characteristic of a healthy organism, allostasis can contribute to physical costs if the organism must endure challenges too frequently, fails to habituate to repeated exposure to similar stressors, fails to shut off physiological responses after the stressor is over, or can no longer mount an adequate stress response. These physical costs can result in elevated blood pressure, elevated inflammatory cytokine production due to inadequate glucocorticoid levels, or sustained exposure of body tissue to glucocorticoids [103]. The cumulative physical costs of allostasis are termed allostatic load.
Stress appraisals have consistently demonstrated activation of two specific pathways: the HPA axis, which culminates in the secretion of cortisol, and the locus coeruleus/norepinephrine-sympathetic system, which secretes epinephrine and norepinephrine. Disrupted HPA axis functioning in PTSD could play a role in the development and progression of cardiovascular [23] and metabolic disorders [105]. In addition, coeruleus/norepinephrine-sympathetic system activity is often elevated in PTSD and has been associated with cardiovascular abnormalities [103]. Conceptualized within the allostatic load framework, disruption of these systems contributes to cardiovascular and metabolic disease through the cumulative effects on body systems. Continued overactivation of the HPA axis has, for example, been associated with various conditions including memory impairment, hypertension, osteoporosis, insulin resistance, and CVD [106].
Influences of Hostility and Health Behaviors on Cardiovascular/Metabolic Disease in PTSD
The clinical significance of atherosclerotic CVD and type 2 diabetes is becoming a topic of interest in individuals with PTSD. However, mechanisms underlying the relationship of PTSD to CVD and type 2 diabetes are not well understood. In non-psychiatric populations, several mechanisms have been proposed, including personality factors (e.g., hostility), behavioral and lifestyle factors, and biological pathways. As this body of evidence accumulates, a more detailed understanding of the process by which PTSD might influence cardiovascular/metabolic disease becomes increasingly valuable. Some research has provided evidence of tertiary factors that might serve to mediate or moderate the conditions under which PTSD is associated with cardiovascular/metabolic disease. Of these factors, hostility has emerged as a promising construct for contributing to a more complete understanding of this relationship.
Hostility
Meta-analytic, epidemiological, and laboratory studies have shown that individuals with PTSD have higher hostility than those without PTSD [107–109]. In addition, PTSD intensity has been positively correlated with hostility level [110]. Furthermore, in some but not all PTSD studies [111–113], hostility and anger have been negatively associated with treatment response [114]. For this reason, the variability and potential predictive role of hostility in people with PTSD are of potential clinical significance. For our purposes, hostility is defined as a broad construct [4, 115] comprising cognitive components such as negative beliefs about others; affective components such as anger, annoyance, resentment, disgust, and contempt; and behavioral components ranging from subtle interpersonal conflicts to overt physical aggression to more subtle conflicts with others.
A large body of research on hostility has noted an association with adverse health outcomes [2, 4, 8, 33, 38, 108, 115–126]. These outcomes include more severe coronary artery disease, peripheral artery disease, and hypertension; lower physician ratings of functional health; and premature mortality from all causes [121, 127, 128]. Hostility has been shown to be a risk factor for poor health, including an association with increased rates of hypertension and higher levels of lipids in both men and women [129–142] and increased risk of coronary heart disease and myocardial infarction [118, 119, 143–148]. Even when traditional risk factors (e.g., smoking, cholesterol levels) are controlled, hostility is a significant predictor of all-cause mortality [139, 144, 149]. Angry feelings have been shown to be related to CVD risk factors [150, 151] and to predict the risk for development of metabolic syndrome in women [152].
Several recent studies have complemented previous research on hostility and increased risk of cardiovascular/metabolic disease by noting a link between hostility and altered physiological reactivity. For example, male participants in one study who were “quarrelsome” exhibited decreased PNS activity during social interactions [153], a finding that parallels studies of impaired PNS reactivity in PTSD. Previous research with police officers reporting varying levels of PTSD symptoms noted a more than twofold increase in flow-mediated dilation following occlusive pressure in officers with low levels of PTSD symptoms, relative to those with severe PTSD symptoms [154]. This finding suggests poor reactivity of the vasculature and provides a potential mechanism for links between PTSD and CVD. Some research has found that individuals with PTSD exhibit greater cardiovascular responsivity when reliving an anger-provoking event from their past [115]. However, another study observed that hostile individuals who received a good apology had quick recoveries of both heart rate and blood pressure [141]. This study is particularly interesting, because recent research suggests that it is the prolonged duration of the physiological response, rather than an exaggerated initial reactivity, that is strongly associated with elevated trait hostility [155].
The physiological reactivity associated with hostility may also be related to social environment. In a study of couple interactions, the physiological reactivity of women high in hostility varied as a function of their husbands’ hostility. Women with husbands who were low in hostility exhibited low baseline heart rate and blood pressure but exaggerated reactivity to conflict. In contrast, when both the husband and wife were high in hostility, the women were elevated on baseline cardiovascular measures, but exhibited less reactivity to conflict [156]. These findings imply that environmental variables could contribute to sustained changes in physiological indices, even when trait hostility remains elevated. Modification of physiological reactions in hostile people provides encouraging evidence of the possibility of effective intervention.
As with research on medical illness in PTSD, relatively few studies have investigated relationships between hostility and medical illness in women [157]. In one study of women, hostility was related to increased intima-media thickness of the carotid artery wall, suggestive of the early stages of atherosclerosis [158]. Insulin resistance and fasting insulin level have also been significantly associated with hostility, but only in women [159].
Research into the influence of hostility on the relationship between PTSD and cardiovascular/metabolic health has yet to definitively identify the precise role of hostility. Hostility could be useful in identifying the conditions under which PTSD might be related to cardiovascular and/or metabolic disease. Research indicating that only people with PTSD who are high in hostility are at increased risk of disease would support an interpretation of hostility as a moderator, while studies observing that PTSD is associated with increased risk of cardiovascular/metabolic disease through its effects on hostility would support the conceptualization of hostility as a mediator. In addition to acting as a full mediator hostility could also partially mediate the relationship between PTSD and cardiovascular/metabolic disease. A partial mediation model is similar to the full mediation model in that PTSD would alter hostility levels, which would in turn alter risk of cardiovascular/metabolic disease. However, in a partial mediation model, hostility would only explain a portion of the increased risk of cardiovascular/metabolic disease, with the remaining effects being independent of hostility. Gottdiener and colleagues [160] assessed people without any coronary artery disease, congestive heart failure, or diabetes. A mental stress task was associated with significantly diminished flow-mediated arterial vasodilation. Those high in hostility exhibited enhanced effects of mental stress on flow-mediated dilation. In this case, higher trait hostility was a condition under which flow-mediated dilation was further diminished, though no test of moderation was conducted. There has generally been little investigation into how anger and hostility relate to health outcomes among those with PTSD; however, Ouimette and colleagues [161] found that anger/hostility was significantly associated with disease among only those with PTSD [162]. Several additional studies have found a relationship between hostility and cardiovascular activity among only people with PTSD [115]. In a recent study of women with and without PTSD, increases in hostility were associated with greater increases in heart rate suggesting that hostility may moderate the effect of PTSD on cardiovascular outcomes [163].
As reviewed above, there is evidence that both individuals high in hostility and individuals with PTSD have increased sympathetic nervous system hyperreactivity [73, 116, 128, 138–140, 149, 150, 164–169]. Indeed, hostility may account for the hyperreactivity observed in people with PTSD. The well-established links between hostility and SNS hyperreactivity and chronic illness support the relevance of hostility to the association of PTSD with cardiovascular/metabolic disease. A few studies have examined PTSD symptom clusters to explore relationships with physical illness. In one study, symptom severity on the hyperarousal cluster, which includes irritability, was related to a higher concentration of precoagulant factors in the vasculature [162]. An analysis of data from a large sample of Vietnam veterans with PTSD found no association between dissociative symptoms and a number of physiological symptoms, including heart rate, skin conductance, electromyographic data, and blood pressure [170]. Another study comparing 20 male Croatian combat veterans to non-PTSD males matched on age and BMI found no differences in blood platelet function [171]. However, the authors cautioned that this study was designed as a pilot study with low power to detect group differences. Taken together, the research to date suggests that hostility may interact with PTSD to increase cardiovascular and metabolic risk factors and that this interaction may contribute to increased risk of poorer health outcomes and cardiovascular mortality.
Depression
Research has consistently found that symptoms of depression are common in PTSD populations. In addition, a number of studies have linked depression with CVD [172]. In fact, the research on depression and CVD serves as a valuable model for PTSD research, as initial observational studies of individuals with pre-existing CVD were followed by community studies of individuals without established CVD [172]. Because MDD is often comorbid with PTSD [173, 174], examination of this comorbidity is likely to reveal important information in the risk for adverse physical health outcomes in those with PTSD.
Several studies have observed an association between depression and biomarkers of CVD risk, such as reduced 24-h HRV [175, 176] and endothelial dysfunction [177]. These findings indicate that depression needs to be considered as a potential confounder or effect modifier in studies of PTSD. A few studies have investigated the influence of depression on the relationship between PTSD and physical disease. For example, controlling for depression, PTSD has been associated with a twofold increase in self-reported CVD in American Indians [21]. In contrast, the association of MDD with CVD was not significant after accounting for both traditional risk factors and PTSD [21]. Data from civilian women suggest that while controlling for depression attenuates the relationship between PTSD and CVD, women with PTSD still suffer from higher rates of CVD [22]. Concordant with data on CVD, available evidence links depression with elevated levels of blood glucose [159, 178, 179] and risk of diabetes [180, 181]. Therefore, it is important that research on the relationships between PTSD and cardiovascular/metabolic disease address the issue of comorbid depression.
To effectively evaluate this relationship, it will be essential to measure depression and account for its influence in studies of PTSD and cardiovascular/metabolic illness. In addition, intervention research could compare people with PTSD only to those with PTSD and comorbid depression. Finally, future research must consider the potential impact of low socioeconomic status and social isolation, which are associated with not only PTSD and depression, but also increased risk for CVD [172].
Health Risk Behaviors
A number of behaviors may contribute to the increased risk for poorer health in individuals with PTSD. For example, a number of studies have documented a tendency for PTSD patients to engage in increased alcohol use, higher BMI, and greater rates of smoking [7, 8, 182–185]. Studies of both men and women have noted an association between hostility and reports of higher BMI [186, 187] and poorer health habits including less physical exercise, less self-care, more alcohol use [188–191], more cigarette smoking [134, 191–193], and greater caloric intake [187].
Only a few studies have investigated the effects of PTSD on cardiovascular/metabolic disease separately from the effects of behavioral health habits. A study of patients with substance use disorders found that those with PTSD had significantly increased rates of CVD [194]. This effect was independent of gender and ethnicity. In a study comparing patients from a VA rehabilitation unit with PTSD to demographically matched inpatients with alcohol dependence, those with PTSD were reported to have a higher frequency of CVD and diabetes [195]. Although the alcohol dependence group had more cigarette smoking pack years, the PTSD group had higher blood cholesterol and triglyceride levels and higher rates of obesity [195]. These findings suggest that increased risk of cardiovascular/metabolic disease in PTSD may be independent of alcohol intake, but these results might be explained by other behavioral health factors. Future research would benefit from consideration of behavioral health differences when investigating physical health in PTSD.
Conclusions and Future Directions
Limitations to Current Evidence
Despite a number of important findings, the research on PTSD and cardiovascular and metabolic disorders has several important issues to address to more completely evaluate a possible link. To complement the literature with male veterans, epidemiologic studies of PTSD and physical illness must continue to establish that results are consistent across demographic groups, such as age, race, gender, veteran status, socioeconomic status, and marital status. There is also a need for longitudinal data [22] and large, prospective epidemiologic trials with established clinical assessments of PTSD and medical disorders [3].
While the focus of this review has been on the development of cardiovascular and metabolic disease in people who already have PTSD, pre-clinical markers and disease sometimes precede the onset of PTSD. Several studies have documented the development of PTSD following life-threatening events that accompany CVD, such as myocardial infarction, as well as life-threatening events that accompany metabolic disease, such as hyperglycemic episodes. Longitudinal research can address the question of temporal precedence by following people with PTSD without pre-clinical markers of cardiovascular/metabolic disease to determine whether they are more likely to develop disease markers or the disease itself than people without PTSD. Longitudinal research might also enable investigation of the distinct influences of comorbid psychological disorders on pre-clinical disease markers, as well as provide information on associations between PTSD and pre-clinical disease markers before the onset of other disorders.
While the relative contributions of genetic factors and PTSD to the development of cardiovascular/metabolic disorders have not been established, it is possible that there may be genetic factors that predispose individuals to both conditions, and these may account for some or all of the association between PTSD and cardiovascular/metabolic disease. Furthermore, although this review has examined influences of PTSD on cardiovascular/metabolic disease, it is possible that traumatic experiences increase the risk of disease even in individuals who do not develop PTSD. Risk of medical illness could increase with injuries sustained during the trauma or infections occurring during the trauma or treatment. Much of the research into this topic has compared people with PTSD to similarly traumatized, non-PTSD individuals to consider the effects of PTSD independently. This methodology has strengthened the understanding of health effects in PTSD and will be an important part of future research.
It would be helpful to have pre-trauma physical health information to determine whether PTSD played a role in the development of a given medical illness and/or whether the illness could have resulted from shared risk factors (e.g., socioeconomic status) for PTSD and medical problems. Due to the relative lack of research available on a possible link between PTSD and metabolic disorders, there needs to be much more research to account for the influences of the many contributors to metabolic health risk in people with PTSD. Disease biomarkers of potential interest include autonomic dysregulation, endothelial dysfunction, metabolic syndrome, and insulin resistance.
Future studies could evaluate whether PTSD with or without comorbid MDD is associated with pre-clinical markers of cardiovascular risk. Promising pre-clinical markers include vascular endothelial function, 24-h heart rate variability, and baroreflex sensitivity. Considering that individuals with both PTSD and high hostility are at a higher risk for poor cardiovascular health, future research could also extend existing findings on third variables in the relationship between PTSD and risk of cardiovascular/metabolic disease in order to determine whether there is an interaction between PTSD and hostility. Analyses could evaluate whether PTSD is related to insulin resistance and whether levels of hostility moderate this relationship. Inclusion of both a PTSD without comorbid MDD and a PTSD with comorbid MDD group will allow for direct evaluation of the effect of PTSD, and the potential additive effect of MDD.
Although a number of studies have examined the relationship between traumatization and health outcomes, there are no studies to date evaluating multiple precursor measures of CVD and diabetes in a younger PTSD sample. Given that much of this evidence has been collected in older samples, the literature in this area would be strengthened by careful assessment of PTSD and biomarkers of CVD and type 2 diabetes in a younger, trauma-exposed sample.
Pre-trauma Vulnerability
The research literature has not fully established the extent to which reactivity to aversive stimuli is the result of PTSD, rather than a pre-trauma vulnerability factor. While some evidence suggests that measures such as pre-trauma eyeblink startle and skin conductance under contextual threat predict the development of PTSD symptoms, similar predictive power has not been evident with regard to pre-trauma heart rate response to contextual threat [196]. Orr and colleagues [49] have shown that individuals with PTSD have an exaggerated heart rate response to startle relative to that of their healthy monozygotic twins. Twins research offers evidence that trauma exposure and PTSD alter physiological responses to trauma cues; however, there is also evidence that genetic factors play a role in PTSD vulnerability [197, 198]. Specifically, recent twins research points to increased vulnerability in individuals with neurological abnormalities such as subtle neurological dysfunction (also known as neurological soft signs), decreased hippocampal volume, and abnormal cavum septum pellucidum [199]. While these variables probably represent pre-trauma risk factors, a substantial literature documents that cardiovascular, particularly heart rate, reactivity to trauma cues often results from the development of PTSD.
It is important to note the traits that put individuals at risk for suffering trauma could also contribute to increased risk of cardiovascular or metabolic disease. For example, there is evidence that trauma exposure rates are higher in men, younger people, and members of minority groups residing in urban areas [200]. In addition to demographic predictors, elevated trauma exposure rates have been documented in people with traits such as neuroticism and extraversion, as well as pre-existing psychiatric disorders [201]. The presence of these potential confounders in PTSD and health research underscore the importance of longitudinal designs, diverse study samples, and assessment of relevant pre-trauma characteristics in future research.
Prevention of Cardiovascular and Metabolic Disease in PTSD
Given the high prevalence of PTSD [202] and the accumulating evidence of an accompanying medical morbidity and mortality [203], there is a need for research identifying specific intervention targets for reducing the medical burden in PTSD, especially when PTSD becomes chronic. The literature on PTSD and cardiovascular disease indicates that increased monitoring of pre-clinical markers of CVD is warranted. Although the current state of research on metabolic disorders is more equivocal, patients with PTSD might also benefit from increased screening of pre-clinical markers such as lipids and blood glucose levels.
As a complement to increased preventive medical care in those with PTSD, it will be important to implement and augment screening and treatment of psychiatric disorders in medical settings. Considering the frequency with which patients with psychiatric disorders present in primary care rather than mental health facilities [204], screening in medical settings has the potential to be quite beneficial.
While PTSD treatment has clear implications for psychiatric well-being, the implications for physical health are not established. In the absence of treatment, autonomic nervous system activation by trauma cues may be particularly difficult to control for people with PTSD. Experimental findings suggest that when expecting an aversive event, people with PTSD exhibit exaggerated eyeblink responses that are not ameliorated either by cues warning of the timing of aversive stimuli or by cues indicating that the aversive event will not occur [196]. However, a number of studies suggest that physiological abnormalities in PTSD remit partially or completely during treatment sessions [205, 206] or after successful PTSD pharmacological [207, 208] or psychotherapeutic [36, 209–212] treatment, as well as combination treatment [210]. One potential clinical implication of the heart rate response to trauma cues was described in a case study detailing the use of heart rate data during psychotherapy to confirm that patients are not engaging in avoidance during exposure and as part of the measures of in-session and between-session habituation to trauma cues [213].
Mechanisms of Links between PTSD and Physical Illness
As reviewed above, the allostatic load model of PTSD and physical illness proposed by Friedman and McEwen [103] is useful in characterizing the onset and progression of cardiovascular and metabolic disease in PTSD. More broadly, the allostatic load model also includes a number of body systems and physiological processes that combine to produce physical illness in people with PTSD. Other reviews have highlighted links between PTSD and chronic inflammation [214]. In addition, disrupted HPA and SAM axis activity likely contributes to medical morbidity in PTSD [5]. Although not reviewed here, significant alterations in physical health could also result from stress-related abnormalities of the immune system, growth hormone production, or release of neuropeptide Y [103].
Although PTSD is typically conceptualized as a mediator of the relationship between trauma and physical disease, longitudinal studies are needed to determine which variables may serve as moderators or mediators in the relationship between PTSD and cardiovascular/metabolic outcomes. For example, if an effect of a predictor on an outcome variable is to be mediated by a third variable, it is conceptually reasonable that the predictor and mediator would precede the outcome temporally. Longitudinal research could provide clarity to mediation models by establishing the temporal precedence necessary to infer causality [215]. As a complement to research on mediation, moderation models suggested by research reporting associations of PTSD with cardiovascular/metabolic disease outcomes that vary at different levels of a third variable could also be valuable in identifying intervention targets and groups. In addition to overt phenotype characteristics such as gender, genetic research could prove valuable in identifying groups of individuals with PTSD who are at risk of cardiovascular/metabolic disease.
Summary
Although a relatively large literature supports a general link between PTSD and cardiovascular abnormalities, further investigation is needed in several areas. Relatively few studies have investigated metabolic disease and biomarkers as they relate to PTSD diagnosis. The extant evidence suggests a relationship between PTSD and metabolic abnormalities, but inconsistencies prevent definitive conclusions in this area. Both fields of research would benefit from longitudinal designs in diverse samples. In addition, much of the existing literature has based PTSD diagnosis on self-report measures or medical record reviews rather than more valid interview-based PTSD assessments. Furthermore, it will continue to be important to consider the impact of comorbid disorders such as depression, substance misuse, and behavioral health problems that could influence the development of physical illness in PTSD. Finally, given the large body of research documenting associations between hostility and physical illness, both generally and in PTSD, it will be important to more fully understand the role of various mediators and moderators in explaining the association between PTSD and physical illness. Clinical implications of this research area are significant in that early detection and prevention as well as possible integrated physical and mental health treatment could substantially modify long-term trajectories in individuals with chronic PTSD. Continued research in this area is necessary to help guide prevention and treatment efforts aimed toward reducing morbidity and mortality in those with PTSD.
References
Beckham JC, Moore SD, Feldman ME, Hertzberg MA, Kirby AC, Fairbank JA. Health status, somatization, and severity of posttraumatic stress disorder in Vietnam combat veterans with posttraumatic stress disorder. Am J Psychiatry. 1998; 155: 1565–1569.
Boscarino JA. Diseases among men 20 years after exposure to severe stress: Implications for clinical research and medical care. Psychosom Med. 1997; 59: 605–614.
Qureshi SU, Pyne JM, Magruder KM, Schulz PE, Kunik ME. The link between post-traumatic stress disorder and physical comorbidities: A systematic review. Psychiatr Q. 2009; 80: 87–97.
Beckham JC, Calhoun PS, Glenn DM, Barefoot JC. Posttraumatic stress disorder, hostility, and health in women: A review of current research. Ann Behav Med. 2002; 24: 219–228.
Boscarino JA. Posttraumatic stress disorder and physical illness: Results from clinical and epidemiologic studies. Ann N Y Acad Sci. 2004; 1032: 141–153.
Beckham JC, Moore SD, Reynolds V. Interpersonal hostility and violence in Vietnam combat veterans with chronic posttraumatic stress disorder: A review of theoretical models and empirical evidence. Aggress Violent Behav. 2000; 5: 451–466.
Friedman MJ, Schnurr PP. The relationship between trauma, PTSD, and physical health. In: Friedman MJ, Charney DS, Deutch AY, eds. Neurobiological and clinical consequences of stress: From normal adaptation to PTSD. New York: Lippincott-Raven; 1995: 507–524.
Schnurr PP, Jankowski MK. Physical health and post-traumatic stress disorder: Review and synthesis. Semin Clin Neuropsychiatry. 1999; 4: 295–304.
Sibai AM, Fletcher AF, Armenian HK. Variations in the impact of long-term wartime stressors on mortality among the middle-aged and older population in Beirut, Lebanon, 1983–1993. Am J Epidemiol. 2001; 154: 128–137.
Kang HK, Bullman TA, Taylor JW. Risk of selected cardiovascular diseases and posttraumatic stress disorder among former World War II prisoners of war. Ann Epidemiol. 2006; 16(5): 381–386.
Lauterbach D, Vora R, Rakow M. The relationship between posttraumatic stress disorder and self-reported health problems. Psychosom Med. 2005; 67: 939–947.
Boscarino JA, Chang J. Electrocardiogram abnormalities among men with stress-related psychiatric disorders: Implications for heart disease and clinical research. Ann Behav Med. 1999; 21: 227–234.
Seng JS, Clark MK, McCarthy AM, Ronis DL. PTSD and physical comorbidity among women receiving Medicaid: Results from service-use data. J Trauma Stress. 2006; 19: 45–56.
Kubzansky LD, Koenen KC, Spiro A, Vokonas PS, Sparrow D. Prospective study of posttraumatic stress disorder symptoms and coronary heart disease in the Normative Aging Study. Arch Gen Psychiatry. 2007; 64: 109–116.
Boscarino JA. Posttraumatic stress disorder and mortality among U.S. Army veterans 30 years after military service. Ann Epidemiol. 2006; 16: 248–256.
O’Toole BI, Catts SV. Trauma, PTSD, and physical health: An epidemiological study of Australian Vietnam veterans. J Psychosom Res. 2008; 64: 33–40.
Dirkzwager AJE, Van der Velden PG, Grievink L, Yzermans CJ. Disaster-related posttraumatic stress disorder and physical health. Psychosom Med. 2007; 69: 435–440.
Kibler JL, Joshi K, Ma M. Hypertension in relation to posttraumatic stress disorder and depression in the US national comorbidity survey. Behav Med. 2009; 34: 125–131.
Santić Z, Lukić A, Sesar D, Milicević S, Ilakovac V. Long-term follow-up of blood pressure in family members of soldiers killed during the war in Bosnia and Herzegovina. Croat Med J. 2006; 47(3): 416–423.
McFarlane AC, Atchison M, Rafalowicz E, Papay P. Physical symptoms in post-traumatic stress disorder. J Psychosom Res. 1994; 38: 715–726.
Sawchuk CN, Roy-Byrne P, Goldberg J, et al. The relationship between posttraumatic stress disorder, depression, and cardiovascular disease in an American Indian tribe. Psychol Med. 2005; 35: 1785–1794.
Kubzansky LD, Koenen KC, Jones AM, Eaton CA. A prospective study of posttraumatic stress disorder symptoms and coronary heart disease in women. Health Psychol. 2009; 28: 125–130.
Boscarino JA. Psychobiologic predictors of disease mortality after psychological trauma: Implications for research and clinical surveillance. J Nerv Ment Dis. 2008; 196: 100–107.
Boscarino JA. A prospective study of PTSD and early-age heart disease mortality among Vietnam veterans: Implications for surveillance and prevention. Psychosom Med. 2008; 70: 668–676.
Flood AM, Boyle SH, Calhoun PS, et al. Prospective study of externalizing and internalizing subtypes of posttraumatic stress disorder and their relationship to mortality in Vietnam veterans. Compr Psychiat. in press.
Weisberg RB, Bruce SEM, J.T., Kessler R, Culpepper L, Keller MB. Nonpsychiatric illness among primary care patients with trauma histories and posttraumatic stress disorder. Psychiatr Serv. 2002; 53: 848–854.
Norman SB, Means-Christenson AJ, Craske MG, Sherbourne CD, Roy-Byrne P, Stein MB. Associations between psychological trauma and physical illness in primary care. J Trauma Stress. 2006; 19: 461–470.
Seng JS, Graham-Bermann SA, Clark MK, McCarthy AM, Ronis DL. Posttraumatic stress disorder and physical comorbidity among female children and adolescents: Results from service-use data. Pediatrics. 2005; 116: 767–776.
Goodwin RD, Davidson JR. Self-reported diabetes and posttraumatic stress disorder among adults in the community. Prev Med. 2005; 40: 570–574.
Sareen J, Cox BJ, Stein MB, Afifi TO, Fleet C, Asmundson GJ. Physical and mental comorbidity, disability, and suicidal behavior associated with posttraumatic stress disorder in a large community sample. Psychosom Med. 2007; 69: 242–248.
Blechert J, Michael T, Grossman P, Lajtman M, Wilhelm FH. Autonomic and respiratory characteristics of posttraumatic stress disorder and panic disorder. Psychosom Med. 2007; 69: 935–943.
Shalev A, Bleich A, Ursano RJ. Posttraumatic stress disorder: Somatic comorbidity and effort tolerance. Psychosom. 1990; 31: 197–203.
Buckley TC, Kaloupek DG. A meta-analytic examination of basal cardiovascular activity in posttraumatic stress disorder. Psychosom Med. 2001; 63: 585–594.
Newton TL, Parker BC, Ho IK. Ambulatory cardiovascular functioning in healthy postmenopausal women with victimization histories. Biol Psychol. 2005; 70: 121–130.
Jones-Alexander J, Blanchard EB, Hickling EJ. Psychophysiological assessment of youthful motor vehicle accident survivors. Appl Psychophysiol Biofeedback. 2005; 30: 115–123.
Rabe S, Zöllner T, Maercker A, Karl A. Cardiovascular correlates of motor vehicle accident related posttraumatic stress disorder and its successful treatment. Appl Psychophysiol Biofeedback. 2006; 31: 315–330.
Hinton D, So V, Pollack MH, Pitman RK, Orr SP. The psychophysiology of orthostatic panic in Cambodian refugees attending a psychiatric clinic. J Psychopathol Behav Assess. 2004; 26: 1–13.
Pole N. The psychophysiology of posttraumatic stress disorder: A meta-analysis. Psychol Bull. 2007; 133: 725–746.
Orr SP, Metzger LJ, Pitman RK. Psychophysiology of post-traumatic stress disorder. Psychiatr Clin North Am. 2002; 25: 271–293.
Greenland P, Daviglus ML, Dyer AR, et al. Resting heart rate is a risk factor for cardiovascular and noncardiovascular mortality: The Chicago Heart Association Detection Project in Industry. Am J Epidemiol. 1999; 149: 853–862.
Veazey CH, Blanchard EB, Hickling EJ, Buckley TC. Physiological responsiveness of motor vehicle accident survivors with chronic posttraumatic stress disorder. Appl Psychophysiol Biofeedback. 2004; 29(1): 51–62.
Obilom RE, Thacher TD. Posttraumatic stress disorder following ethnoreligious conflict in Jos, Nigeria. J Interpers Violence. 2008; 23: 1108–1119.
Lindauer RJ, Booij J, Habraken JB, et al. Effects of psychotherapy on regional cerebral blood flow during trauma imagery in patients with post-traumatic stress disorder: A randomized clinical trial. Psychol Med. 2007; 38: 543–554.
Buckley TC, Holohan D, Greif JL, Bedard M, Suvak M. Twenty-four-hour ambulatory assessment of heart rate and blood pressure in chronic PTSD and non-PTSD veterans. J Trauma Stress. 2004; 17: 163–171.
Forneris CA, Butterfield MI, Bosworth HB. Physiological arousal among women veterans with and without posttraumatic stress disorder. Mil Med. 2004; 4: 307–312.
Muraoka MY, Carlson JG, Chemtob CM. Twenty-four-hour ambulatory blood pressure and heart rate monitoring in combat-related posttraumatic stress disorder. J Trauma Stress. 1998; 3: 473–484.
Beckham JC, Feldman ME, Barefoot JC, et al. Ambulatory cardiovascular activity in Vietnam combat veterans with and without posttraumatic stress disorder. J Consult Clin Psychol. 2000; 68: 269–276.
Beckham JC, Taft CT, Vrana SR, et al. Ambulatory monitoring and physical health report in Vietnam veterans with and without chronic posttraumatic stress disorder. J Trauma Stress. 2003; 16: 329–335.
Orr SP, Metzger LJ, Lasko NB, et al. Physiologic responses to sudden loud tones in monozygotic twins discordant for combat exposure: Association with posttraumatic stress disorder. Arch Gen Psychiatry. 2003; 60: 283–288.
Griffin MG. A prospective assessment of auditory startle alterations in rape and physical assult survivors. J Trauma Stress. 2008; 21: 91–99.
Elsesser K, Sartory G, Tackenberg A. Attention, heart rate, and startle response during exposure to trauma-relevant pictures: A comparison of recent trauma victims and patients with posttraumatic stress disorder. J Abnorm Psychol. 2004; 113(2): 289–301.
Bremner JD, Vythilingam M, Vermetten E, et al. Cortisol response to a cognitive stress challenge in posttraumatic stress disorder (PTSD) related to childhood abuse. Psychoneuroendocrinology. 2003; 28: 733–750.
Casada JH, Roache JD. Dissociation of physiology and behavior in PTSD. Int J Psychophysiol. 2006; 62(2): 243–248.
Cuthbert BN, Lang PJ, Strauss C, Drobes D, Patrick CJ, Bradley MM. The psychophysiology of anxiety disorder: Fear memory imagery. Psychophysiology. 2003; 40(3): 407–422.
Peckerman A, Dahl K, Chemitiganti R, LaManca JJ, Ottenweller JE, Natelson BH. Effects of posttraumatic stress disorder on cardiovascular stress responses in Gulf War veterans with fatiguing illness. Auton Neurosci. 2003; 108(1–2): 63–72.
Kibler JL, Lyons JA. Perceived coping ability mediates the relationship between PTSD severity and heart rate recover in veterans. J Trauma Stress. 2004; 17: 23–29.
Carson MA, Metzger LJ, Lasko NB, et al. Physiologic reactivity to startling tones in female Vietnam nurse veterans with PTSD. J Trauma Stress. 2007; 20: 657–666.
Wessa M, Flor H. Failure of extinction of fear responses in posttraumatic stress disorder: Evidence from second-order conditioning. Am J Psychiatry. 2007; 164: 1684–1692.
Ginsberg JP, Ayers E, Burriss L, Powell DA. Disruption of bradycardia associated with discriminative conditioning in combat veterans with PTSD. Neuropsychiatric Disease and Treatment. 2008; 4(3): 635–646.
Muhtz C, Wester M, Yassouridis A, Widemann K, Kellner M. A combined dexamethasone/corticotropin-releasing hormone test in patients with chronic PTSD—first preliminary results. J Psychiatr Res. 2008; 42: 689–693.
Geracioti TD Jr, Baker DG, Kasckow JW, et al. Effects of trauma-related audiovisual stimulation on cerebrospinal fluid norepinephrine and corticotropin-releasing hormone concentrations in post-traumatic stress disorder. Psychoneuroendocrinology. 2008; 33: 416–424.
Strawn JR, Ekhator NN, Horn PS, Baker DG, Geracioti TD Jr. Blood pressure and cerebrospinal fluid norepinephrine in combat-related posttraumatic stress disorder. Psychosom Med. 2004; 66: 757–759.
Schmahl CG, Elzinga BM, Ebner UW, et al. Psychophysiological reactivity to traumatic and abandonment scripts in borderline personality and posttraumatic stress disorders: A preliminary report. Psychiatr Res. 2004; 126: 33–42.
Kellner M, Yassouridix A, Hubner R, Baker DG, Wiedermann K. Endocrine and cardiovascular responses to corticotropin-releasing hormone in patients with posttraumatic stress disorder: A role for atrial natriuretic peptide? Neuropsychobiology. 2003; 47: 102–108.
Schnurr PP, Spiro A, Paris AH. Physician-diagnosed medical disorders in relation to PTSD symptoms in older male military veterans. Health Psychol. 2000; 19: 91–97.
Luscher TF. Mack Forster Award Lecture: The endothelium as a target and mediator of cardiovascular disease. Eur J Clin Invest. 1993; 23: 670–685.
Harrison DG. Endothelial dysfunction in atherosclerosis. Basic Res Cardiol. 1994; 89: 87–102.
Celermajer DS, Sorenson KE, Gooch VM, et al. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet. 1992; 340: 1111–1115.
Celermajer DS, Sorenson KE, Bull C, Robinson JH, Deanfield JE. Endothelium-dependent dilation in the systemic arteries of asymptomatic subjects relates to coronary risk factors and their interaction. J Am Coll Cardiol. 1994; 24: 1468–1474.
Violanti JM, Andrew M, Burchfiel CM, Hartley TA, Charles LE, Miller DB. Post-traumatic stress symptoms and cortisol patterns among police officers. Policing: An International Journal of Police Strategies & Management. 2007; 30(2): 189–202.
Von Kanel R, Hepp U, Traber R, et al. Measures of endothelial dysfunction in plasma of patients with posttraumatic stress disorder. Psychiatr Res. 2008; 158: 363–373.
Von Kanel R, Hepp U, Buddeberg C, et al. Altered blood coagulation in patients with posttraumatic stress disorder. Psychosom Med. 2006; 68: 598–604.
Sloan RP, Shapiro PA, Bagiella E, Myers MM, Gormon JM. Cardiac autonomic control buffers blood pressure variability responses to challenge: A psychophysiologic model of coronary artery disease. Psychosom Med. 1999; 61: 58–68.
Sloan RP, Demeersman RE, Shapiro PA, et al. Cardiac autonomic control is inversely related to blood pressure variability responses to psychological challenge. Am J Physiol. 1997; 272: H2227–H2232.
Cohen H, Kotler M, Matar MA, Kaplan Z, Miodownik H, Cassuto Y. Power spectral analysis of HR variability in posttraumatic stress disorder patients. Biol Psychiatry. 1997; 4: 627–629.
Hughes JW, Dennis MF, Beckham JC. Baroreceptor sensitivity at rest and during stress in women with posttraumatic stress disorder or major depressive disorder. J Trauma Stress. 2007; 20(5): 667–676.
Hughes JW, Feldman ME, Beckham JC. Posttraumatic stress disorder is associated with attenuated baroreceptor sensitivity among female, but not male, smokers. Biol Psychol. 2006; 71: 296–302.
Scheeringa MS, Zeanah CH, Myers L, Putnam F. Heart period and variability findings in preschool children with posttraumatic stress symptoms. Biol Psychiatry. 2004; 55: 685–691.
Ulmer CS, Calhoun PS, Edinger JD, Wagner HR, Beckham JC. Sleep disturbance and baroreceptor sensitivity in women with posttraumatic stress disorder. J Traum Stress. 2009; 22: 643–647.
Rich MW, Saini JS, Kleiger RE, Carney RM, TeVelde A, Freedland KE. Correlation of heart rate variability with clinical and angiography variables and late mortality after coronary angiography. Am J Cardiol. 1988; 62: 714–717.
Kleiger RE, Miller JP, Bigger JT, Moss AJ. Decreased heart rate variability and its association with increased mortality after acute myocardial infarction. Am J Cardiol. 1987; 59: 256–262.
Bilchick KC, Fetics B, Djoukeng R, et al. Prognostic value of heart rate variability in chronic congestive heart failure (Veterans Affairs’ Survival Trial of Antiarrhythmic Therapy in Congestive Heart Failure). Am J Cardiol. 2002; 90: 24–28.
Hopper JW, Spinazzola J, Simpson WB, Van der Kolk B. Preliminary evidence of parasympathetic influence on basal heart rate in posttraumatic stress disorder. J Psychosom Res. 2006; 60: 83–90.
Bigger JT, Fleiss JL, Rolnitzky LM, Steinman RC. The ability of several short-term measures of RR variability to predict mortality after myocardial infarction. Circulation. 1993; 88: 927–934.
Brosschot JF, Van Dijk E, Thayer JF. Daily worry is related to low heart rate variability during waking and the subsequent nocturnal sleep period. Int J Psychophysiol. 2007; 63: 39–47.
Hayano J, Jiang Y, Waugh R, O’Connor C, Frid D, Blumenthal JA. Stability over time of circadian rhythm of variability of heart rate in patients with stable coronary artery disease. Am Heart J. 1997; 134: 411–418.
Bonnet MH, Arand DL. EEG arousal norms by age. J Clin Sleep Med. 2007; 3: 271–274.
Haley RW, Vongpatanasin W, Wolfe GI, et al. Blunted circadian variation in autonomic regulations of sinus node function in veterans with Gulf War syndrome. Am J Med. 2004; 117: 469–478.
Sack M, Hopper JW, Lamprecht F. Low respiratory sinus arrhythmia and prolonged psychophysiological arousal in posttraumatic stress disorder: Heart rate dynamics and individual differences in arousal regulation. Biol Psychiatry. 2004; 55(3): 284–290.
Bedi US, Arora R. Cardiovascular manifestations of posttraumatic stress disorder. J Natl Med Assoc. 2007; 99(6): 642–649.
Masi CB, Hawkley LC, Rickett EM, Cacioppo JT. Respiratory sinus arrhythmia and diseases of aging: Obesity, diabetes mellitus, and hypertension. Biol Psychol. 2007; 74: 212–223.
Lehtinen J, Uusitupa M, Siitonen O, Pyorala K. Prevalence of neuropathy in newly diagnosed NIDDM and non diabetic control subjects. Diabetes. 1989; 38: 1307–1313.
Pfeifer MA, Weinberg CR, Cook D, et al. Autonomic neural dysfunction in recently diagnosed diabetic subjects. Diabetes Care. 1984; 7: 447–453.
Toyry JP, Niskanen LK, Mantysaari MJ, Lansimies EA, Uusitupa M. Occurrence predictors and clinical significance of autonomic neuropathy in NIDDM: Ten-year follow-up from the diagnosis. Diabetes. 1996; 45: 308–315.
Verrotti A, Chiarelli F, Morgese G. Autonomic dysfunction in newly diagnosed insulin-dependent diabetes mellitus children. Pediatr Neurol. 1996; 14: 49–52.
Watkins LL, Blumenthal JA, Davidson JRT, Babyak MA, McCants CB Jr, Sketch MH Jr. Phobic anxiety, depression, and risk of ventricular arrhythmias in patients with coronary heart disease. Psychosom Med. 2006; 68: 651–656.
Kudat H, Akkaya B, Sozen AB, et al. Heart rate variability in diabetes patients. J Int Med Res. 2006; 34: 291–296.
Solter V, Thaller V, Karlovic D, Crnkovic D. Elevated serum lipids in veterans with combat-related chronic posttraumatic stress disorder. Croat Med J. 2002; 43: 685–689.
Trief PM, Ouimette P, Wade M, Shanahan P, Weinstock RS. Post-traumatic stress disorder and diabetes: Co-morbidity and outcomes in a male veterans sample. J Behav Med. 2006; 29: 411–418.
Karlovic D, Buljan D, Martinac M, Marcinko D. Serum lipid concentrations in Croatian veterans with posttraumatic stress disorder, posttraumatic stress disorder comorbid with major depressive disorder, or major depressive disorder. J Korean Med Sci. 2004; 19: 431–436.
Karlovic D, Martinac M, Buljan D, Zoricic Z. Relationship between serum lipid concentrations and posttraumatic stress disorder symptoms in soldiers with combat experiences. Acta Med Okayama. 2004; 58: 23–27.
Maia DB, Marmar C, Mendlowicz MV, et al. Abnormal serum lipid profile in Brazilian police officers with post-traumatic stress disorder. J Affect Disord. 2008; 107: 259–263.
Friedman MJ, McEwen BS. Posttraumatic stress disorder, allostatic load, and medical illness. In: Schnurr PP, Green BL, eds. Trauma and Health: Physical Health Consequences of Exposure to Extreme Stress. Washington, DC: American Psychological Association; 2004: 507–524.
McEwen BS. Protective and damaging effects of stress mediators. N Engl J Med. 1998; 338(3): 171–179.
Reagan LP, Grillo CA, Piroli GG. The As and Ds of stress: Metabolic, morphological and behavioral consequences. Eur J Pharmacol. 2008; 585: 64–75.
Chrousos GP, Gold PW. Editorial: A healthy body in a healthy mind—and vice versa—the damaging power of ‘uncontrollable stress’. J Clin Endocrinol. 1998; 56: 1842–1845.
Evans S, Giosan C, Patt I, Spielman L, Difede J. Anger and its association to distress and social/occupational functioning in symptomatic disaster relief works responding to the September 11, 2001, World Trade Center disaster. J Trauma Stress. 2006; 19: 147–152.
Orth U, Wieland E. Anger, hostility and posttraumatic stress disorder in trauma-exposed adults: A meta-analysis. J Consult Clin Psychol. 2006; 74: 698–706.
Jakupcak M, Conybeare D, Phelps L, et al. Anger, hostility, and aggression among Iraq and Afghanistan War veterans reporting PTSD and subthreshold PTSD. J Trauma Stress. 2007; 20: 945–954.
Begic D, Jokic-Begic N. Heterogeneity of posttraumatic stress disorder symptoms in Croatian war veterans: Retrospective study. Croat Med J. 2007; 48: 133–139.
Cahill SP, Rauch SA, Hembree EA, Foa EB. Effect of cognitive-behavioral treatment for PTSD on anger. Journal of Cognitive Psychotherapy: An International Quarterly. 2003; 17: 113–131.
Taylor E. Outcome predictors for three PTSD treatments: Exposure, EMDR, and relaxation training. Journal of Cognitive Psychotherapy: An International Quarterly. 2003; 17: 149–162.
van Minnen A, Arntz A, Keijsers GP. Prolonged exposure in patients with chronic PTSD: Predictors of treatment outcome and dropout. Behav Res Ther. 2002; 40: 439–457.
Stapleton JA, Taylor S, Asmundson GJG. Effects of three PTSD treatments on anger and guilt: Exposure therapy, eye movement desensitization and reprocessing, and relaxation training. J Trauma Stress. 2006; 19: 19–28.
Beckham JC, Vrana SR, Barefoot JC, Feldman ME, Fairbank JA, Moore SM. Magnitude and duration of cardiovascular responses to anger in Vietnam veterans with and without posttraumatic stress disorder. J Consult Clin Psychol. 2002; 70: 228–234.
Shapiro D, Goldstein IB, Jamner LD. Effects of clinical hostility, anger out, and defensiveness on ambulatory blood pressure in black and white college students. Psychosom Med. 1996; 58: 354–364.
Enkelman HCH, Bishop GD, Tong EMW, et al. The relationship of hostility, negative affect and ethnicity to cardiovascular responses: An ambulatory study in Singapore. Int J Psychophysiol. 2005; 56: 185–197.
Shapiro D, Jamner LD, Goldstein IB. Daily mood states and ambulatory blood pressure. Psychophysiology. 1997; 34: 399–405.
Adams SH, Cartwright LK, Ostrove JM, Stewart AJ, Wink P. Psychological predictors of good health in three longitudinal studies of educated midlife women. Health Psychol. 1998; 17: 412–420.
Calhoun PS, Bosworth HB, Siegler IC, Bastian LA. The relationship between hostility and behavioral risk factors for poor health in women veterans. Prev Med. 2001; 33: 552–557.
Miller TQ, Smith TW, Turner CW, Guijarro ML, Hallet AJ. A meta-analytic review of research on hostility and physical health. Psychol Bull. 1996; 119: 322–348.
Butterfield MI, Forneris CA, Feldman ME, Beckham JC. Hostility and functional health status in women veterans with posttraumatic stress disorder versus women with other primary psychiatric diagnosis: A preliminary study. J Trauma Stress. 2000; 13: 735–741.
Kilpatrick DB, Resick PA, Veronen LJ. Effects of a rape experience: A longitudinal study. J Soc Issues. 1981; 37: 1–17.
Kulka RA, Schlenger WE, Fairbank JA, et al. Trauma and the Vietnam War Generation: Report of Findings from the National Vietnam Veterans Readjustment Study. New York: Brunner/Mazel; 1990.
Zlotnick C, Zakriski AL, Shea MT, Costello E. The long-term sequelae of sexual abuse: Support for a complex posttraumatic stress disorder. J Trauma Stress. 1996; 9: 195–205.
Blake DD, Weathers FW, Nagy LM, et al. The development of a clinician-administered posttraumatic stress disorder scale. J Trauma Stress. 1995; 8: 75–80.
Barefoot JC, Lipkus IM. The assessment of anger and hostility. In: Siegman AW, Smith TW, eds. Anger, Hostility, and the Heart. Hillsdale: Lawrence Erlbaum; 1994: 67–96.
Smith TW. Hostility and health: Current status of a psychosomatic hypothesis. Health Psychol. 1992; 11: 139–150.
Dujovne VF, Houston BK. Hostility-related variables and plasma lipid levels. J Behav Med. 1991; 14: 555–565.
Knox SS, Jacobs DR Jr, Chesney MA, Raczynski J, McCreath H. Psychosocial factors and plasma lipid levels in black and white young adults: The Coronary Artery Risk in Young Adults Study data. Psychosom Med. 1996; 58: 365–373.
Lane JD, Pieper CF, Barefoot JC, Williams RB, Siegler I. Caffeine and cholesterol: Interactions with hostility. Psychosom Med. 1994; 56: 260–266.
Lundberg U, Hedman M, Melin B, Frankenhaeuser M. Type A behavior in healthy males and females as related to physiological reactivity and blood lipids. Psychosom Med. 1989; 51: 113–122.
Pope ML, Smith TW. Cortisol excretion in high and low cynically hostile men. Psychosom Med. 1991; 53: 386–392.
Siegler I, Peterson BL, Barefoot JC, Williams RB. Hostility during late adolescence predicts coronary risk factors at mid-life. Am J Epidemiol. 1992; 136: 146–154.
Suarez EC, Bates MP, Harralson TL. The relation of hostility to lipids and lipoproteins in women: Evidence for the role of antagonistic hostility. Ann Behav Med. 1998; 20: 59–63.
Vogele C. Serum lipid concentrations, hostility and cardiovascular reactions to mental stress. Int J Psychophysiol. 1998; 28: 167–179.
Weidner G, Boughal T, Connor SL, Pieper CF, Mendell NR. Relationship of job strain to standard coronary risk factors and psychological characteristics in women and men of the Family Heart Study. Health Psychol. 1997; 16: 239–247.
Nelson TL, Palmer RF, Pedersen NL. The metabolic syndrome mediates the relationship between cynical hostility and cardiovascular disease. Exp Aging Res. 2004; 30: 163–177.
Zhang J, Niaura R, Todaro JF, et al. Suppressed hostility predicted hypertension incidence among middle-aged men: The normative aging study. J Behav Med. 2005; 28: 443–454.
Zhang J, Niaura R, Dyer JR, et al. Hostility and urine norepinephrine interact to predict insulin resistance: The VA Normative Aging Study. Psychosom Med. 2006; 68: 718–726.
Anderson DE, Metter EJ, Hougaku H, Najjar SS. Suppressed anger is associated with increased carotid arterial stiffness in older adults. Am J Hypertens. 2006; 19: 1129–1134.
Krantz DS, Olson SB, Francis JL, et al. Anger, hostility, and cardiac symptoms in women with suspected coronary artery disease: The Women’s Ischemia Syndrome Evaluation (WISE) Study. J Womens Health. 2006; 15: 1214–1223.
Adams SH. Role of hostility in women’s health during mid-life: A longitudinal study. Health Psychol. 1994; 13: 488–495.
Barefoot JC, Larsen S, Lieth LVD, Schroll M. Hostility, incidence of acute myocardial infarction, and mortality in a sample of older Danish men and women. Am J Epidemiol. 1995; 142: 477–484.
Cartwright LK, Wink P, Kmetz C. What leads to good health in midlife women physicians? Some clues from a longitudinal study. Psychosom Med. 1995; 57: 284–292.
Knox SS, Siegmund KD, Weidner G, Ellison RC, Adelman A, Paton C. Hostility, social support, and coronary heart disease in the National Heart, Lung, and Blood Institute Family Heart Study. Am J Cardiol. 1998; 82: 1192–1196.
Lahad A, Heckbert SR, Koepsell TD, Psaty BM, Patrick DL. Hostility, aggression, and the risk of nonfatal myocardial infarction in postmenopausal women. J Psychosom Res. 1997; 43: 183–195.
Matthews KA, Owens JF, Kuller LH, Sutton-Tyrrell K, Jansen-McWilliams L. Are hostility and anxiety associated with carotid atherosclerosis in healthy postmenopausal women? Psychosom Med. 1998; 60: 633–638.
Smith TW, Glazer K, Ruiz JM, Gallo LC. Hostility, anger, aggressiveness, and coronary heart disease: An interpersonal perspective on personality, emotion, and health. J Pers. 2004; 76: 1217–1270.
Raikkonen K, Matthews KA, Kuller LH, Reiber C, Bunker CH. Anger, hostility and visceral adipose tissue in healthy postmenopausal women. Metabolism. 1999; 48: 1146–1152.
Raikkonen K, Mathews KA, Kuller LH. Trajectory of psychological risk and incident hypertension in middle-aged women. Hypertension. 2001; 38: 798–802.
Raikkonen K, Mathews KA, Kuller LH. The relationship between psychological risk attributes and the metabolic syndrome in healthy women: Antecedent or consequence? Metabolism. 2002; 51: 1573–1577.
D’Antono B, Moskowitz DS, Miners C, Archambault J. Gender and communal trait differences in the relations among social behaviour, affect arousal, and cardiac autonomic control. J Behav Med. 2005; 28: 267–279.
Violanti JM, Andrew ME, Burchfiel CM, Dorn J, Hartley T, Miller DB. Posttraumatic stress symptoms and subclinical cardiovascular disease in police officers. Int J Stress Manag. 2006; 13: 541–554.
Anderson CM, Linden W, Habra ME. The importance of examining blood pressure reactivity and recovery in anger provocation research. Int J Psychophysiol. 2005; 57: 159–163.
Broadwell SD, Light KC. Hostility, conflict and cardiovascular response in married couples: A focus on the dyad. Int J Behav Med. 2005; 12: 142–152.
Everson-Rose SA, Lewis TT. Psychosocial factors and cardiovascular diseases. Annu Rev Public Health. 2005; 26: 469–500.
Everson-Rose SA, Lewis TT, Karavolos K, Matthews KA, Sutton-Tyrrell K, Powell LH. Cynical hostility and carotid atherosclerosis in African American and white women: The Study of Women’s Health Across the Nation (SWAN) Heart Study. Am Heart J. 2006; 152(5): 982e7–982e13.
Suarez EC. Sex differences in the relation of depressive symptoms, hostility, and anger expression to indices of glucose metabolism in nondiabetic adults. Health Psychol. 2006; 25: 484–492.
Gottdiener JS, Kop WJ, Hausner E, McCeney MK, Herrington D, Krantz DS. Effects of mental stress on flow-mediated brachial arterial dilation and influence of behavioral factors and hypercholesterolemia in subjects without cardiovascular disease. Am J Cardiol. 2003; 92(6): 687–691.
Ouimette P, Cronkite R, Henson BR, Prins A, Gima K, Moos RH. Posttraumatic stress disorder and health status among female and male medical patients. J Trauma Stress. 2004; 17: 1–9.
Beckham JC, Flood AM, Dennis MF, Calhoun PS. Ambulatory cardiovascular activity and hostility ratings in women with chronic posttraumatic stress disorder. Biol Psychiatry. 2009; 65: 268–272.
Ouimette P, Cronkite R, Prins A, Moos RH. Posttraumatic stress disorder, anger and hostility, and physical health status. J Nerv Ment Dis. 2004; 192(8): 563–566.
Benotsch EG, Christensen AJ, McKelvey L. Hostility, social support and ambulatory cardiovascular activity. J Behav Med. 1997; 20: 163–176.
Christensen AJ, Smith TW. Cynical hostility and cardiovascular reactivity during self-disclosure. Psychosom Med. 1993; 55: 193–202.
Houston BK. Anger, hostility, and psychophysiological reactivity. In: Siegman AW, Smith TW, eds. Anger, Hostility, and the Heart. Hillsdale: Lawrence Erlbaum; 1994: 97–115.
Suarez EC, Kuhn CM, Schanberg SM, Williams RB, Zimmering EA. Neuroendocrine, cardiovascular, and emotional responses of hostile men: The role of interpersonal challenge. Psychosom Med. 1998; 60: 78–88.
Williams RB, Barefoot JC, Shekelle RB. The health consequences of hostility. In: Chesney MA, Rosenman RH, eds. Anger and Hostility in Cardiovascular and Behavioral Disorders. Washington, DC: Hemisphere; 1985: 173–185.
Brosschot JF, Thayer JF. Anger inhibition, cardiovascular recovery, and vagal function: A model of the link between hostility and cardiovascular disease. Ann Behav Med. 1998; 20: 326–332.
Kaufman ML, Kimble MO, Kaloupek DG, et al. Peritraumatic dissociation and physiological response to trauma-relevant stimuli in Vietnam combat veterans with posttraumatic stress disorder. J Nerv Ment Dis. 2002; 190(3): 167–174.
Vidović A, Vilibić M, Markotić A, et al. Baseline level of platelet-leukocyte aggregates, platelet CD63 expression, and soluble P-selectin concentration in patients with posttraumatic stress disorder: A pilot study. Psychiatr Res. 2007; 150(2): 211–216.
Ford DE. Depression, trauma, and cardiovascular health. In: Schnurr PP, Green BL, eds. Trauma and Health: Physical Consequences of Exposure to Extreme Stress. Washington, DC: American Psychological Association; 2004: 73–98.
Kessler RC, Chiu WT, Demler O, Walters EE. Prevalence, severity and comorbidity of 12-month DSM-IV Disorders in the National Comorbidity Survey replication. Arch Gen Psychiatry. 2005; 62: 617–627.
Keane TM, Kaloupek DG. Comorbid psychiatric disorders in PTSD: Implications for research. Ann N Y Acad Sci. 1997; 821: 24–34.
Carney RM, Blumenthal JA, Freedland KE, et al. Low heart rate variability and the effect of depression on post-myocardial infarction mortality. Arch Intern Med. 2005; 165: 2024–2028.
Krittayaphong R, Cascio WE, Light KC, et al. Heart rate variability in patients with coronary artery disease: Differences in patients with higher and lower depression scores. Psychosom Med. 1997; 59: 231–235.
Sherwood A, Hinderliter AL, Watkins LL, Waugh RA, Blumenthal JA. Impaired endothelial function in coronary heart disease patients with depressive symptomatology. J Am Coll Cardiol. 2005; 46: 656–659.
Boyle SH, Surwit RS, Georgiades A, et al. Depressive symptoms, race, and glucose concentrations. Diabetes Care. 2007; 30(10): 2484–2488.
McCaffery JM, Niaura R, Todaro JF, Swan GE, Carmelli D. Depressive symptoms and metabolic risk in adult male twins enrolled in the National Heart, Lung, and Blood Institute Twin Study. Psychosom Med. 2003; 65: 490–497.
Carnethon MR, Kinder LS, Fair JM, Stafford RS, Fortmann SP. Symptoms of depression as a risk factor for incident diabetes: Findings from the National Health and Nutrition Examination Epidemiologic Follow-Up Study, 1971–1992. Am J Epidemiol. 2003; 158: 416–423.
Everson-Rose SA, Torrens JI, Meyer PM, et al. Depressive symptoms, insulin resistance and risk of diabetes in women at midlife. Diabetes Care. 2004; 27: 2856–2862.
Beckham JC. Smoking and anxiety in combat veterans with chronic posttraumatic stress disorder: A review. J Psychoactive Drugs. 1999; 31: 103–110.
Schnurr PP, Green BL, eds. Trauma and Health: Physical Health Consequences of Exposure to Extreme Stress. Washington, DC: American Psychological Association; 2004.
Fu S, McFall M, Saxon AJ, et al. Posttraumatic stress disorder and smoking: A systematic review. Nicotine Tob Res. 2007; 9: 1071–1084.
Beckham JC, Dennis MF, McClernon FJ, Mozley SL, Collie CF, Vrana SR. The effects of cigarette smoking on script-driven imagery in smokers with and without posttraumatic stress disorder. Addict Behav. 2007; 32: 2900–2915.
Houston BK, Vavak CR. Hostility: Developmental factors, psychosocial correlates, and health behaviors. Health Psychol. 1991; 10: 9–17.
Scherwitz LW, Perkins LL, Chesney MA, Hughes GH, Sidney S, Manalio TA. Hostility and health behaviors in young adults: The CARDIA study. Am J Epidemiol. 1992; 136: 136–145.
Leiker M, Hailey BJ. A link between hostility and disease: Poor health habits? Behav Med. 1988; 3: 129–133.
Miller TQ, Markides KS, Chiriboga DA, Ray LA. A test of the psychosocial vulnerability and health behavior models of hostility: Results from an 11-year follow-up study of Mexican Americans. Psychosom Med. 1995; 57: 572–581.
Sidney S, Sternfeld B, Haskell WL, Jacobs DR Jr, Chesney MA, Hulley SB. Television viewing and cardiovascular risk factors in young adults: The CARDIA study. Ann Epidemiol. 1996; 6: 154–159.
Whiteman MC, Fowkes FG, Deary IJ, Lee AJ. Hostility, cigarette smoking and alcohol consumption in the general population. Soc Sci Med. 1997; 44: 1089–1096.
Koskenvuo M, Kaprio J, Rose RJ, et al. Hostility as a risk factor for mortality and ischemic heart disease in men. Psychosom Med. 1988; 50: 330–340.
Shekelle RB, Gale M, Ostfeld AM, Paul O. Hostility, risk of coronary disease and mortality. Psychosom Med. 1983; 45: 109–114.
Ouimette P, Goodwin E, Brown PJ. Health and well being of substance use disorder patients with and without posttraumatic stress disorder. Addict Behav. 2006; 31: 1415–1423.
David D, Woodward C, Esquenazi J, Mellman TA. Comparison of comorbid physical illnesses among veterans with PTSD and veterans with alcohol dependence. Psychiatr Serv. 2004; 55: 82–85.
Pole N, Neylan TC, Otte C, Henn-Hasse C, Metzler TJ, Marmar CR. Prospective prediction of posttraumatic stress disorder symptoms using fear potentiated auditory startle responses. Biol Psychiatry. 2009; 65: 235–240.
True WR, Rice J, Eisen SA, Heath AC. A twin study of genetic and environmental contributions to liability for posttraumatic stress symptoms. Arch Gen Psychiatry. 1993; 50(4): 257–265.
Koenen KC, Anstadter AB, Nugent NR. Gene-environment interaction in posttraumatic stress disorder: An update. J Trauma Stress. 2009; 22(5): 416–426.
Pitman RK, Gilbertson MW, Gurvits TV, et al. Clarifying the origin of biological abnormalities in PTSD through the study of identical twins discordant for combat exposure. Ann N Y Acad Sci. 2006; 1071: 242–254.
Breslau N, Kessler R, Chilcoat H, Schultz L, Davis G, Andreski P. Trauma and posttraumatic stress disorder in the community: The 1996 Detroit Area Survey of Trauma. Arch Gen Psychiatry. 1998; 55: 626–632.
Breslau N. Epidemiologic studies of trauma, posttraumatic stress disorder, and other psychiatric disorders. Can J Psychiatry. 2002; 47: 923–929.
Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey replication. Arch Gen Psychiatry. 2005; 62: 593–602.
Boscarino JA. External-cause mortality after psychologic trauma: The effects of stress exposure and predisposition. Compr Psychiatry. 2006; 47: 503–514.
Norquist GS, Regier DA. The epidemiology of psychiatric disorders and the de facto mental health care system. Annu Rev Med. 1996; 47: 473–479.
Elofsson UOE, von Schèele B, Theorell T, Söndergaard HP. Physiological correlates of eye movement desensitization and reprocessing. J Anxiety Disord. 2008; 22: 622–634.
Sack M, Lempa W, Steinmetz A, Lamprecht F, Hofmann A. Alterations in autonomic tone during trauma exposure using eye movement desensitization and reprocessing (EMDR)-Results of a preliminary investigation. J Anxiety Disord. 2008; 22: 1264–1271.
Brunet A, Orr SP, Tremblay J, Robertson K, Nader K, Pitman RK. Effect of post-retrieval propranolol on psychophysiologic responding during subsequent script-driven traumatic imagery in post-traumatic stress disorder. Journal of Psychiatry Research. 2008; 42: 503–506.
Tucker P, Beebe KL, Burgin C, et al. Paroxetine treatment of depression with posttraumatic stress disorder: Effects on autonomic reactivity and cortisol secretion. J Clin Psychopharmacol. 2004; 24: 131–140.
Blanchard EB, Hickling EJ, Veazey CH, et al. Treatment-related changes in cardiovascular reactivity to trauma cues in motor vehicle accident-related PTSD. Behavior Therapy. 2002; 33(3): 417–426.
Finch SJ, van Zyl LT. Cardioversion of persistent atrial arrhythmia after treatment with venlafaxine in successful management of major depression and posttraumatic stress disorder. Psychosom. 2006; 47: 533–536.
Mitani S, Fujita M, Sakamoto S, Shirakawa T. Effect of autogenic training on cardiac autonomic nervous activity in high-risk fire service workers for posttraumatic stress disorder. J Psychosom Res. 2006; 60: 439–444.
Nishith P, Duntley SP, Domitrovich PP, Uhles ML, Cook BJ, Stein PK. Effect of cognitive behavioral therapy on heart rate variability during REM sleep in female rape victims with PTSD. J Trauma Stress. 2003; 16: 247–250.
Nishith P, Griffin MG. Utility of the heart rate response as an index of emotional processing in a female rape victim with posttraumatic stress disorder. Cogn Behav Pract. 2002; 9: 302–307.
Von Kanel R, Hepp U, Kraemer B, et al. Evidence for low-grade systemic proinflammatory activity in patients with posttraumatic stress disorder. J Psychiatr Res. 2007; 41: 744–752.
MacKinnon DP, Fairchild AJ, Fritz MS. Mediation analysis. Annu Rev Psychol. 2007; 58: 593–614.
Acknowledgments
This work was primarily supported by 2R01MH62482, but also by 2K24DA016388, 2R01CA081595, HL61784, HL72390, the Office of Research and Development Clinical Science, Department of Veterans Affairs, and the Mid-Atlantic Mental Illness Research Education and Clinical Center. The views expressed in this manuscript are those of the authors and do not necessarily represent the views of the funding agencies. The authors do not have any competing interests that might be interpreted as influencing the research.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Dedert, E.A., Calhoun, P.S., Watkins, L.L. et al. Posttraumatic Stress Disorder, Cardiovascular, and Metabolic Disease: A Review of the Evidence. ann. behav. med. 39, 61–78 (2010). https://doi.org/10.1007/s12160-010-9165-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12160-010-9165-9