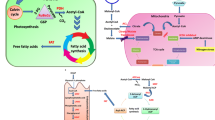
Abstract
Microalgae have been reported to exhibit mutualistic interactions with other microorganisms like bacteria, filamentous fungi, and yeast and help each other co-exist. The potential of microalgae to perform photosynthesis and accumulate lipids make them suitable candidates for lipid production. Biofuel production from various single oleaginous microorganisms is already in practice. However, the high cost of biomass harvesting, extraction of lipids, and contamination issues are significant challenges of biofuel bioprocess commercialization. Recent microalgal co-culture studies showed considerable potential for easy biomass harvesting and reduction in overall energy consumption cost. Therefore, microalgal co-culture could be an alternative to overcome these constraints and enhance biomass and lipid production. Additionally, the integration of the nutrient sequestration process from potential agro-industrial wastewater using microalgal co-culture can reduce the cost of the substrate requirement for cultivation as well as ecological load. The co-culture in wastewater has shown excellent total phosphate removal efficiencies by microalgae Chlorella sorokiniana and yeast Rhodotorula glutinis, nitrogen removal by microalgae C. sorokiniana with activated sludge, and ammonium-nitrogen removal by C. vulgaris and fungi Aspergillus sp. co-culture. This review summarized the current advances towards biofuel and its value-added production from various microalgae co-culture and compared it with monoculture fermentation. It also includes some critical challenges of co-culturing for the economically viable bioprocess development for biofuel production. Furthermore, techno-economic analysis and life-cycle assessment of co-culture technology were also discussed for biofuel production feasibility from microalgal co-culture.


Similar content being viewed by others
Abbreviations
- AMDI:
-
American Dye Manufacturing Institute
- ASP:
-
Aquatic Species Program
- BOD:
-
Biological oxygen demand
- COD:
-
Chemical oxygen demand
- DO:
-
Dissolved oxygen
- DOC:
-
Dissolved organic carbon
- DOE:
-
Department of Energy
- DW:
-
Dry weight
- EPSs:
-
Extracellular polymeric substances
- FAMEs:
-
Fatty acid methyl esters
- FFA:
-
Free fatty acids
- HRAPs:
-
High rate algal ponds
- NH4+-N:
-
Ammonium-nitrogen
- NO3 −-N:
-
Nitrate-nitrogen
- PHAs:
-
Polyhydroxyalkanoic acids
- PUFA:
-
Polyunsaturated fatty acids
- RSM:
-
Response surface methodology
- SCO:
-
Single cell oil
- TAGs:
-
Triacylglycerides
- TN:
-
Total nitrogen
- TOC:
-
Total organic carbon
- TP:
-
Total phosphate
- TSS:
-
Total suspended solids
- VFAs:
-
Volatile fatty acids
- VSS:
-
Volatile suspended solids
References
Zhu LD, Li ZH (2016) Hiltunen E (2016) Strategies for lipid production improvement in microalgae as a biodiesel feedstock. Biomed Res Int. https://doi.org/10.1155/2016/8792548
Liu X (2020) Microbial Technology for the Sustainable Development of Energy and Environment. Biotechnol Rep. https://doi.org/10.1016/j.btre.2020.e00486
International Energy Association (2019) Global energy demand rose by 2.3% in 2018, its fastest pace in the last decade. Available at: https://www.iea.org/newsroom/news/2019/march/global-energy-demand-rose-by-23-in-2018-its-fastest-pace-in-the-last-decade.html. Accessed 26 March 2019
Ramakrishnan VM, Muthukumarasamy N, Balraju P, Pitchaiya S, Velauthapillai D, Pugazhendhi A (2020) Transformation of TiO2 nanoparticles to nanotubes by simple solvothermal route and its performance as dye-sensitized solar cell (DSSC) photoanode. Int J Hydrogen Energ 45:15441–15452
Ahorsu R, Medina F, Constantí M (2018) Significance and challenges of biomass as a suitable feedstock for bioenergy and biochemical production: A review. Energies 11(12):3366
Ho SH, Zhang C, Tao F, Zhang C, Chen WH (2020) Microalgal Torrefaction for Solid Biofuel Production. Trends Biotechnol 38(9):1023–1033. https://doi.org/10.1016/j.tibtech.2020.02.009
Patel A, Karageorgou D, Rova E, Katapodis P, Rova U, Christakopoulos P, Matsakas L (2020) An overview of potential oleaginous microorganisms and their role in biodiesel and omega-3 fatty acid-based industries. Microorganisms 8(3):434. https://doi.org/10.3390/microorganisms8030434
Behera BK, Varma A (2016) Microbial resources for sustainable energy. Springer, Berlin
Kumar A, Ogita S, Yau YY (2018) Biofuels: Greenhouse Gas Mitigation and Global Warming: Next Generation Biofuels and Role of Biotechnology. Springer, Berlin
Ganesana R, Manigandanb S, Samuelc MS, Shanmuganathand R, Brindhadevie K, Chie NTL, Duc AP (2020) A review on prospective production of biofuel from microalgae. Biotechnol Rep:e00509. https://doi.org/10.1016/j.btre.2020.e00509
Mathimani T, Pugazhendhi A (2019) Utilization of algae for biofuel, bio-products and bio-remediation. Biocatal Agric Biotechnol 17:326–330. https://doi.org/10.1016/j.bcab.2018.12.007
Rakesh S, Karthikeyan S (2019) Co-cultivation of microalgae with oleaginous yeast for economical biofuel production. J Farm Sci 32(2):125–130. https://doi.org/10.13140/RG.2.2.10506.41928
Lian J, Wijffels RH, Smidt H, Sipkema D (2018) The effect of the algal microbiome on industrial production of microalgae. Microb Biotechnol 11(5):806–818. https://doi.org/10.1111/2F1751-7915.13296
Khan MI, Shin JH, Kim JD (2018) The promising future of microalgae: current status, challenges, and optimization of a sustainable and renewable industry for biofuels, feed, and other products. Microb Cell Fact 17(1):36. https://doi.org/10.1186/s12934-018-0879-x
Pacheco AR, Segrè D (2019) A multidimensional perspective on microbial interactions. FEMS Microbiol Lett 366(11):fnz125. https://doi.org/10.1093/femsle/fnz125
Magdouli S, Brar SK, Blais JF (2016) Co-culture for lipid production: advances and challenges. Biomass Bioenerg 92:20–30. https://doi.org/10.1016/j.biombioe.2016.06.003
Ghosh S, Chowdhury R, Bhattacharya P (2016) Mixed consortia in bioprocesses: role of microbial interactions. Appl Microbiol Biot 100(10):4283–4295. https://doi.org/10.1007/s00253-016-7448-1
Yen HW, Chen PW, Chen LJ (2015) The synergistic effects for the co-cultivation of oleaginous yeast Rhodotorula glutinis and microalgae Scenedesmus obliquus on the biomass and total lipids accumulation. Bioresour Technol 184:148–152. https://doi.org/10.1016/j.biortech.2014.09.113
Xu L, Cheng X, Wang Q (2018) Enhanced lipid production in Chlamydomonas reinhardtii by co-culturing with Azotobacter chroococcum. Front Plant Sci 9:741. https://doi.org/10.3389/fpls.2018.00741
Osman MEH, Abo-Shady AM, Elshobary ME, Ghafar MAE, Abomohra AEF (2020) Screening of seaweeds for sustainable biofuel recovery through sequential biodiesel and bioethanol production. Environ Sci Pollut Res 27:32481–32493. https://doi.org/10.1007/s11356-020-09534-1
Younes S, Bracharz F, Awad D, Qoura F, Mehlmer N, Brueck T (2020) Microbial lipid production by oleaginous yeasts grown on Scenedesmus obtusiusculus microalgae biomass hydrolysate. Bioproc Biosyst Eng 43:1629–1638. https://doi.org/10.1007/s00449-020-02354-0
Vasconcelos B, Teixeira JC, Dragone G, Teixeira JA (2019) Oleaginous yeasts for sustainable lipid production—from biodiesel to surf boards, a wide range of “green” applications. Appl Microbiol Biot 103:3651–3667. https://doi.org/10.1007/s00253-019-09742-x
La A (2019) Process development for symbiotic culture of Saccharomyces cerevisiae and Chlorella vulgaris for in situ CO2 mitigation (Doctoral dissertation, Paris Saclay). University of Paris-Saclay, Saint-Aubin, France
Zamalloa C, Gultom SO, Rajendran A, Hu B (2017) Ionic effects on microalgae harvest via microalgae-fungi co-pelletization. Biocatal Agricu Biotechnol 9:145–155. https://doi.org/10.1016/j.bcab.2016.12.007
Ahuja D, Tatsutani M (2009) Sustainable energy for developing countries. SAPI EN. S. Surveys and Perspectives Integrating Environment and Society 2(1) http://journals.openedition.org/sapiens/823
Islam AK, Primandari SR, Yaakob Z (2018) Non-Edible Vegetable Oils as Renewable Resources for Biodiesel Production: South-East Asia Perspective. In Advances in Biofuels and Bioenergy. IntechOpen Ltd, London, U.K. https://doi.org/10.5772/intechopen.73304
Srivastava RK (2019) Bio-Energy production by contribution of effective and suitable microbial system. Mater Sci Energ Technol 2(2):308–318. https://doi.org/10.1016/j.mset.2018.12.007
Li-Beisson Y, Thelen JJ, Fedosejevs E, Harwood JL (2019) The lipid biochemistry of eukaryotic algae. Prog Lipid Res 74:31–68
Mühlroth A, Li K, Røkke G, Winge P, Olsen Y, Hohmann-Marriott MF, Vadstein O, Bones AM (2013) Pathways of lipid metabolism in marine algae, co-expression network, bottlenecks and candidate genes for enhanced production of EPA and DHA in species of Chromista. Mar Drugs 11(11):4662–4697. https://doi.org/10.3390/md11114662
Turkkul B, Deliismail O, Seker E (2020) Ethyl esters biodiesel production from Spirulina sp. and Nannochloropsis oculata microalgal lipids over alumina-calcium oxide catalyst. Renew Energy 145:1014–1019. https://doi.org/10.1016/j.renene.2019.06.093
Liu S, Abu Hajar HA, Riefler G, Stuart BJ (2018) Lipid Extraction from Spirulina sp. and Schizochytrium sp. Using Supercritical CO with Methanol. BioMed Res Int:2018. https://doi.org/10.1155/2018/2720763
Dourou M, Aggeli D, Papanikolaou S, Aggelis G (2018) Critical steps in carbon metabolism affecting lipid accumulation and their regulation in oleaginous microorganisms. Appl Microbiol Biotechnol 102(6):2509-23. 23. https://doi.org/10.1007/s00253-018-8813-z
Velmurugan P, Lee YH, Venil CK, Lakshmanaperumalsamy P, Chae JC, Oh BT (2010) Effect of light on growth, intracellular and extracellular pigment production by five pigment-producing filamentous fungi in synthetic medium. J Biosci Bioeng 109(4):346–350
del Pilar S-SM, Sauceda-Carvajal D, Castro-Ochoa FY, Molina-Cárdenas CA (2020) The Use of Light Spectra to Improve the Growth and Lipid Content of Chlorella vulgaris for Biofuels Production. Bioenergy Res 3:1–2. https://doi.org/10.1007/s12155-019-10070-1
Rayati M, Rajabi Islami H, Shamsaie Mehrgan M (2020) Light Intensity Improves Growth, Lipid Productivity, and Fatty Acid Profile of Chlorococcum oleofaciens (Chlorophyceae) for Biodiesel Production. Bioenergy Res 19:1–1. https://doi.org/10.1007/s12155-020-10144-5
Sharma N, Fleurent G, Awwad F, Cheng M, Meddeb-Mouelhi F, Budge SM, Germain H, Desgagné-Penix I (2020) Red light variation an effective alternative to regulate biomass and lipid profiles in Phaeodactylum tricornutum. Appl Sci 10(7):2531. https://doi.org/10.3390/app10072531
Patil V, Tran KQ, Giselrød HR (2008) Towards sustainable production of biofuels from microalgae. Int J Mol Sci 9(7):1188–1195. https://doi.org/10.3390/ijms9071188
Pal P, Chew KW, Yen HW, Lim JW, Lam MK, Show PL (2019) Cultivation of oily microalgae for the production of third-generation biofuels. Sustainability 11(19):5424. https://doi.org/10.3390/su11195424
Andrade LM, Andrade CJ, Dias M, Nascimento CA, Mendes MA (2018) Chlorella and Spirulina microalgae as sources of functional foods, nutraceuticals, and food supplements; an overview. MOJ Food Process Technol 6(1):45–58. https://doi.org/10.15406/mojfpt.2018.06.00144
Gao K, Orr V, Rehmann L (2016) Butanol fermentation from microalgae-derived carbohydrates after ionic liquid extraction. Bioresour Technol 206:77–85. https://doi.org/10.1016/j.biortech.2016.01.036
Sarker PK, Kapuscinski AR, Bae AY, Donaldson E, Sitek AJ, Fitzgerald DS, Edelson OF (2018) Towards sustainable aquafeeds: Evaluating substitution of fishmeal with lipid-extracted microalgal co-product (Nannochloropsis oculata) in diets of juvenile Nile tilapia (Oreochromis niloticus). PLoS One 13(7):e0201315. https://doi.org/10.1371/journal.pone.0201315
Tevatia R, Allen J, Rudrappa D, White D, Clemente TE, Cerutti H, Demirel Y, Blum P (2015) The taurine biosynthetic pathway of microalgae. Algal Res 9:21–26. https://doi.org/10.1016/j.algal.2015.02.012
Lordan S, Ross RP, Stanton C (2011) Marine bioactives as functional food ingredients: potential to reduce the incidence of chronic diseases. Mar Drugs 9(6):1056–1100. https://doi.org/10.3390/md9061056
Dourou M, Dritsas P, Baeshen MN, Elazzazy A, AL-Farga A, Aggelis G (2020) High-added value products from microalgae and prospects of aquaculture wastewaters as microalgae growth media. FEMS Microbiol Lett 367(12). https://doi.org/10.1093/femsle/fnaa081
Dhanasekaran D, Sundaresan M, Suresh A, Thajuddin N, Thangaraj R, Vinothini G (2017) Oleaginous Microorganisms for Biofuel Development. Environ Sci & Engg Vol. 12: Climate Change and Sustainable Technology
Cho HU, Park JM (2018) Biodiesel production by various oleaginous microorganisms from organic wastes. Bioresour Technol 256:502–508. https://doi.org/10.1016/j.biortech.2018.02.010
Rempel A, Biolchi GN, Antunes AC, Gutkoski JP, Treichel H, Colla LM (2020) Cultivation of Microalgae in Media Added of Emergent Pollutants and Effect on Growth, Chemical Composition, and Use of Biomass to Enzymatic Hydrolysis. Bioenergy Res 2:1–3. https://doi.org/10.1007/s12155-020-10177-w
Valdez-Ojeda RA, del Rayo Serrano-Vázquez MG, Toledano-Thompson T, Chavarría-Hernández JC, Barahona-Pérez LF (2020) Effect of Media Composition and Culture Time on the Lipid Profile of the Green Microalga Coelastrum sp. and Its Suitability for Biofuel Production. Bioenergy Res 30:1–3. https://doi.org/10.1007/s12155-020-10160-5
Malibari R, Sayegh F, Elazzazy AM, Baeshen MN, Dourou M, Aggelis G (2018) Reuse of shrimp farm wastewater as growth medium for marine microalgae isolated from Red Sea–Jeddah. J Clean Prod 198:160–169. https://doi.org/10.1016/j.jclepro.2018.07.037
Dourou M, Tsolcha ON, Tekerlekopoulou AG, Bokas D, Aggelis G (2018) Fish farm effluents are suitable growth media for Nannochloropsis gaditana, a polyunsaturated fatty acid producing microalga. Eng Life Sci 18(11):851–860. https://doi.org/10.1002/elsc.201800064
Li DW, Balamurugan S, Yang YF, Zheng JW, Huang D, Zou LG, Yang WD, Liu JS, Guan Y, Li HY (2019) Transcriptional regulation of microalgae for concurrent lipid overproduction and secretion. Sci Adv 5(1):eaau3795. https://doi.org/10.1126/sciadv.aau3795
Yoo C, Jun SY, Lee JY, Ahn CY, Oh HM (2010) Selection of microalgae for lipid production under high levels carbon dioxide. Bioresour Technol 101(1):S71–S74. https://doi.org/10.1016/j.biortech.2009.03.030
Rodolfi L, Chini Zittelli G, Bassi N, Padovani G, Biondi N, Bonini G, Tredici MR (2009) Microalgae for oil: Strain selection, induction of lipid synthesis and outdoor mass cultivation in a low-cost photobioreactor. Biotechnol Bioeng 102(1):100–112. https://doi.org/10.1002/bit.22033
Illman AM, Scragg AH, Shales SW (2000) Increase in Chlorella strains calorific values when grown in low nitrogen medium. Enzyme Microb Technol 27(8):631–635. https://doi.org/10.1016/S0141-0229(00)00266-0
Cheng Y, Zhou W, Gao C, Lan K, Gao Y, Wu Q (2009) Biodiesel production from Jerusalem artichoke (Helianthus tuberosus L.) tuber by heterotrophic microalgae Chlorella protothecoides. J Chem Technol Biotechnol 84(5):777–781. https://doi.org/10.1002/jctb.2111
Liang Y, Sarkany N, Cui Y (2009) Biomass and lipid productivities of Chlorella vulgaris under autotrophic, heterotrophic and mixotrophic growth conditions. Biotechnol Lett 31(7):1043–1049. https://doi.org/10.1007/s10529-009-9975-7
Wen X, Geng Y, Li Y (2019) Enhanced lipid production in Chlorella pyrenoidosa by continuous culture. Bioresour Technol 161:297–303. https://doi.org/10.1016/j.biortech.2014.03.077
Li Y, Horsman M, Wang B, Wu N, Lan CQ (2008) Effects of nitrogen sources on cell growth and lipid accumulation of green alga Neochloris oleoabundans. Appl Microbiol Biot 81(4):629–636. https://doi.org/10.1007/s00253-008-1681-1
Pleissner D, Lam WC, Sun Z, Lin CS (2013) Food waste as nutrient source in heterotrophic microalgae cultivation. Bioresour Technol 137:139–146. https://doi.org/10.1016/j.biortech.2013.03.088
Cheirsilp B, Thawechai T, Prasertsan P (2017) Immobilized oleaginous microalgae for production of lipid and phytoremediation of secondary effluent from palm oil mill in fluidized bed photobioreactor. Bioresour Technol 241:787–794. https://doi.org/10.1016/j.biortech.2017.06.016
Weldy CS, Huesemann MI (2007) Lipid production by Dunaliella salina in batch culture: effects of nitrogen limitation and light intensity. J Undergrad Res 7:115–122
Takagi M, Yoshida T (2006) Effect of salt concentration on intracellular accumulation of lipids and triacylglyceride in marine microalgae Dunaliella cells. J Biosci Bioeng 101(3):223–226. https://doi.org/10.1263/jbb.101.223
Ratledge C, Wynn JP (2002) The biochemistry and molecular biology of lipid accumulation in oleaginous microorganisms. Adv Appl Microbiol 51:1–52. https://doi.org/10.1016/s0065-2164(02)51000-5
Han L, Pei H, Hu W, Jiang L, Ma G, Zhang S, Han F (2015) Integrated campus sewage treatment and biomass production by Scenedesmus quadricauda SDEC-13. Bioresour Technol 175:262–268. https://doi.org/10.1016/j.biortech.2014.10.100
Shin DY, Cho HU, Utomo JC, Choi YN, Xu X, Park JM (2014) Biodiesel production from Scenedesmus bijuga grown in anaerobically digested food wastewater effluent. Bioresour Technol 184:215–221. https://doi.org/10.1016/j.biortech.2014.10.090
Goswami L, Namboodiri MT, Kumar RV, Pakshirajan K, Pugazhenthi G (2017) Biodiesel production potential of oleaginous Rhodococcus opacus grown on biomass gasification wastewater. Renew Energ 105:400–406. https://doi.org/10.1016/j.renene.2016.12.044
Zhang Q, Li Y, Xia L (2014) An oleaginous endophyte Bacillus subtilis HB1310 isolated from thin-shelled walnut and its utilization of cotton stalk hydrolysate for lipid production. Biotechnol Biofuels 7(1):152 http://www.biotechnologyforbiofuels.com/content/7/1/152
Gouda MK, Omar SH, Aouad LM (2008) Single cell oil production by Gordonia sp. DG using agro-industrial wastes. World J Microb Biot 24(9):1703. https://doi.org/10.1007/s11274-008-9664-z
Gujjala LK, Kumar SJ, Talukdar B, Dash A, Kumar S, Sherpa KC, Banerjee R (2017) Biodiesel from oleaginous microbes: opportunities and challenges. Biofuels 10(1):45–59. https://doi.org/10.1080/17597269.2017.1402587
Matsakas L, Giannakou M, Vörös D (2017) Effect of synthetic and natural media on lipid production from Fusarium oxysporum. Electron J Biotechn 30:95–102. https://doi.org/10.1016/j.ejbt.2017.10.003
Chen J, Zhang X, Tyagi RD, Drogui P (2018) Utilization of methanol in crude glycerol to assist lipid production in non-sterilized fermentation from Trichosporon oleaginosus. Bioresour Technol 253:8–15. https://doi.org/10.1016/j.biortech.2018.01.008
Al-Hawash AB, Li S, Zhang X, Zhang X, Ma F (2018) Productivity of γ-Linoleic acid by oleaginous fungus Cunninghamella echinulata using a pulsed high magnetic field. Food Biosci 21:1–7. https://doi.org/10.1016/j.fbio.2017.10.007
Sitepu IR, Garay LA, Sestric R, Levin D, Block DE, German JB, Boundy-Mills KL (2014) Oleaginous yeasts for biodiesel: current and future trends in biology and production. Biotechnol Adv 32(7):1336–1360. https://doi.org/10.1016/j.biotechadv.2014.08.003
Kleinzeller A (1944) Fat Formation in Torulopsis lipofera. Biochem J 38(5):480
Oguri E, Masaki K, Naganuma T, Iefuji H (2012) Phylogenetic and biochemical characterization of the oil-producing yeast Lipomyces starkeyi. Anton Leeuw Int J G 101(2):359–368. https://doi.org/10.1007/s10482-011-9641-7
Eroshin VK, Krylova NI (1983) Efficiency of lipid synthesis by yeasts. Biotechnol Bioeng 25(7):1693–1700. https://doi.org/10.1002/bit.260250702
Kitcha S, Cheirsilp B (2011) Screening of oleaginous yeasts and optimization for lipid production using crude glycerol as a carbon source. Energy Procedia 9:274–282. https://doi.org/10.1016/j.egypro.2011.09.029
Pan LX, Yang DF, Shao L, Li W, Chen GG, Liang ZQ (2009) Isolation of the oleaginous yeasts from the soil and studies of their lipid-producing capacities. Food Technol Biotech 47(2):215–220
Amaretti A, Raimondi S, Leonardi A, Rossi M (2012) Candida freyschussii: an oleaginous yeast producing lipids from glycerol. Industrial Biotechnology International Conference 2012 27:139–144
Duarte SH, de Andrade CC, Ghiselli G, Maugeri F (2013) Exploration of Brazilian biodiversity and selection of a new oleaginous yeast strain cultivated in raw glycerol. Bioresour Technol 138:377–381. https://doi.org/10.1016/j.biortech.2013.04.004
Franklin S, Piechocki J, Norris LM, Decker SM, Wee J, Zdanis D (2011) Oleaginous yeast food compositions. United States patent application US 13/087,305.
Sentheshanmuganathan S, Nickerson WJ (1962) Composition of cells and cell walls of triangular and ellipsoidal forms of Trigonopsis variabilis. J Microbiol 27(3):451–464. https://doi.org/10.1099/00221287-27-3-451
Bati N, Hammond EG, Glatz BA (1984) Biomodification of fats and oils: Trials with Candida lipolytica. J Am Oil Chem Soc 61(11):1743–1746. https://doi.org/10.1007/BF02582139
Amaretti A, Raimondi S, Sala M, Roncaglia L, De Lucia M, Leonardi A, Rossi M (2010) Single cell oils of the cold-adapted oleaginous yeast Rhodotorula glacialis DBVPG 4785. Microb Cell Fact 9(1):73 http://www.microbialcellfactories.com/content/9/1/73
Sitepu IR, Sestric R, Ignatia L, Levin D, German JB, Gillies LA, Almada LA, Boundy-Mills KL (2013) Manipulation of culture conditions alters lipid content and fatty acid profiles of a wide variety of known and new oleaginous yeast species. Bioresour Technol 144:360–369. https://doi.org/10.1016/j.biortech.2013.06.047
Rossi M, Buzzini P, Cordisco L, Amaretti A, Sala M, Raimondi S, Ponzoni C, Pagnoni UM, Matteuzzi D (2009) Growth, lipid accumulation, and fatty acid composition in obligate psychrophilic, facultative psychrophilic, and mesophilic yeasts. FEMS Microbiol Ecol 69(3):363–372. https://doi.org/10.1111/j.1574-6941.2009.00727.x
Huang C, Chen XF, Xiong L, Ma LL (2012) Oil production by the yeast Trichosporon dermatis cultured in enzymatic hydrolysates of corncobs. Bioresour Technol 110:711–714. https://doi.org/10.1016/j.biortech.2012.01.077
Iassonova DR (2009) Lipid synthesis and encapsulation by Cryptococcus curvatus. J Am Oil Chem Soc 85:711. https://doi.org/10.31274/etd-180810-648
Ling J, De Toledo RA, Shim H (2016) Biodiesel production from wastewater using oleaginous yeast and microalgae. In: Shih K (ed) M.N.V. Prasad. Academic Press, New York, Environmental Materials and Waste, pp 179–212. https://doi.org/10.1016/B978-0-12-803837-6.00008-1
Probst KV, Vadlani PV (2015) Production of single cell oil from Lipomyces starkeyi ATCC 56304 using biorefinery by-products. Bioresour Technol 198:268–275. https://doi.org/10.1016/j.biortech.2015.09.018
Llamas M, Dourou M, González-Fernández C, Aggelis G, Tomás-Pejó E (2020) Screening of oleaginous yeasts for lipid production using volatile fatty acids as substrate. Biomass Bioenerg 138:105553. https://doi.org/10.1016/j.biombioe.2020.105553
Papanikolaou S, Rontou M, Belka A, Athenaki M, Gardeli C, Mallouchos A, Kalantzi O, Koutinas AA, Kookos IK, Zeng AP, Aggelis G (2016) Conversion of biodiesel-derived glycerol into biotechnological products of industrial significance by yeast and fungal strains. Eng Life Sci 17(3):262–281. https://doi.org/10.1002/elsc.201500191
Bellou S, Triantaphyllidou IE, Mizerakis P, Aggelis G (2016) High lipid accumulation in Yarrowia lipolytica cultivated under double limitation of nitrogen and magnesium. J Biotechnol 234:116–126. https://doi.org/10.1016/j.jbiotec.2016.08.001
Arous F, Frikha F, Triantaphyllidou IE, Aggelis G, Nasri M, Mechichi T (2016) Potential utilization of agro-industrial wastewaters for lipid production by the oleaginous yeast Debaryomyces etchellsii. J Clean Prod 133:899–909. https://doi.org/10.1016/j.jclepro.2016.06.040
Dourou M, Kancelista A, Juszczyk P, Sarris D, Bellou S, Triantaphyllidou IE, Rywinska A, Papanikolaou S, Aggelis G (2016) Bioconversion of olive mill wastewater into high-added value products. J Clean Prod 139:957–969. https://doi.org/10.1016/j.jclepro.2016.08.133
Daskalaki A, Perdikouli N, Aggeli D, Aggelis G (2019) Laboratory evolution strategies for improving lipid accumulation in Yarrowia lipolytica. Appl Microbiol Biotechnol 103(20):8585–8596. https://doi.org/10.1007/s00253-019-10088-7
Aloklah B, Alhajali A, Yaziji S (2014) Identification of some yeasts by fatty acid profiles. Pol J Microbiol 63(4):467–472
Fakas S, Papanikolaou S, Galiotou-Panayotou M, Komaitis M, Aggelis G (2008) Organic nitrogen of tomato waste hydrolysate enhances glucose uptake and lipid accumulation in Cunninghamella echinulata. J Appl Microbial 105(4):1062–1070. https://doi.org/10.1111/j.1365-2672.2008.03839.x
Dharumadurai D, Annamalai P, Nooruddin T, Saravanan C, Isolation (2014) characterization of antibacterial methyl substituted β-lactum compound from Streptomyces noursei DPTD21 in saltpan soil, India. JBAPN 4(2):71–88. https://doi.org/10.1080/22311866.2013.833388
Gundolf R, Oberleitner S, Richter J (2019) Evaluation of New Genetic Toolkits and Their Role for Ethanol Production in Cyanobacteria. Energies 12(18):3515. https://doi.org/10.3390/en12183515
Lo J, Zheng T, Hon S, Olson DG, Lynd LR (2015) The bifunctional alcohol and aldehyde dehydrogenase gene, adhE, is necessary for ethanol production in Clostridium thermocellum and Thermoanaerobacterium saccharolyticum. J Bacteriol 197(8):1386–1393. https://doi.org/10.1128/JB.02450-14
Celaya-Herrera S, Casados-Vázquez LE, Valdez-Vazquez I, Barona-Gómez F, Bideshi DK, Barboza-Corona JE (2020) A Cellulolytic Streptomyces sp. Isolated from a Highly Oligotrophic Niche Shows Potential for Hydrolyzing Agricultural Wastes. Bioenergy Res 31:1–1. https://doi.org/10.1007/s12155-020-10174-z
Subhash GV, Mohan SV (2015) Sustainable biodiesel production through bioconversion of lignocellulosic wastewater by oleaginous fungi. Biomass Convers Biorefin 5(2):215–226
Khot M, Kamat S, Zinjarde S, Pant A, Chopade B, RaviKumar A (2012) Single cell oil of oleaginous fungi from the tropical mangrove wetlands as a potential feedstock for biodiesel. Microb Cell Fact 11(1):71. doi:10.1186%2F1475-2859-11-71
Economou CN, Aggelis G, Pavlou S, Vayenas DV (2011) Single cell oil production from rice hulls hydrolysate. Bioresour Technol 102(20):9737–9742. https://doi.org/10.1016/j.biortech.2011.08.025
Moustogianni A, Bellou S, Triantaphyllidou IE, Aggelis G (2015) Feasibility of raw glycerol conversion into single cell oil by zygomycetes under non-aseptic conditions. Biotechnol Bioeng 112(4):827–831. https://doi.org/10.1002/bit.25482
Athenaki M, Gardeli C, Diamantopoulou P, Tchakouteu SS, Sarris D, Philippoussis A, Papanikolaou S (2018) Lipids from yeasts and fungi: physiology, production and analytical considerations. J Appl Microbiol 124(2):336–367. https://doi.org/10.1111/jam.13633
Kikukawa H, Sakuradani E, Ando A, Shimizu S (2018 May 1) Ogawa J (2018) Arachidonic acid production by the oleaginous fungus Mortierella alpina 1S-4: A review. J Adv Res 11:15–22. https://doi.org/10.1016/j.jare.2018.02.003
Sharifyazd S, Karimi K (2017) Effects of fermentation conditions on valuable products of ethanolic fungus Mucor indicus. Electron J Biotechnol 30:77–82. https://doi.org/10.1016/j.ejbt.2017.09.003
Carvalho AK, Bento HB, Rivaldi JD, de Castro HF (2018) Direct transesterification of Mucor circinelloides biomass for biodiesel production: effect of carbon sources on the accumulation of fungal lipids and biofuel properties. Fuel 234:789–796. https://doi.org/10.1016/j.fuel.2018.07.029
Muniraj IK, Xiao L, Hu Z, Zhan X, Shi J (2013) Microbial lipid production from potato processing wastewater using oleaginous filamentous fungi Aspergillus oryzae. Water Res 47(10):3477–3483. https://doi.org/10.1016/j.watres.2013.03.046
Wu MH, Huang SB, Lee GB (2010) Microfluidic cell culture systems for drug research. Lab Chip 10(8):939–956. https://doi.org/10.1039/b921695b
Suvarna K, Lolas A, Patricia Hughes MS, Friedman RL (2011) MICROBIOLOGY-Case Studies of Microbial Contamination in Biologic Product Manufacturing. Am Pharm Rev 14(1):50
Bader J, Mast-Gerlach E, Popović MK, Bajpai R, Stahl U (2010) Relevance of microbial coculture fermentations in biotechnology. J Appl Microbial 109(2):371–387. https://doi.org/10.1111/j.1365-2672.2009.04659.x
Santos CA, Reis A (2014) Microalgal symbiosis in biotechnology. Appl Microbiol Biot 98(13):5839–5846. https://doi.org/10.1007/s00253-014-5764-x
Hays SG, Patrick WG, Ziesack M, Oxman N, Silver PA (2015) Better together: engineering and application of microbial symbioses. Curr Opin Biotechnol 36:40–49. https://doi.org/10.1016/j.copbio.2015.08.008
Younginger BS, Friesen ML (2019) Connecting signals and benefits through partner choice in plant–microbe interactions. FEMS Microbiol Lett 366(18):fnz217.
Krespach MK, García-Altares M, Flak M, Schoeler H, Scherlach K, Netzker T, Schmalzl A, Mattern DJ, Schroeckh V, Komor A, Mittag M (2020) Lichen-like association of Chlamydomonas reinhardtii and Aspergillus nidulans protects algal cells from bacteria. ISME J 14(11):2794–2805
Padmaperuma G, Kapoore RV, Gilmour DJ, Vaidyanathan S (2018) Microbial consortia: a critical look at microalgae co-cultures for enhanced biomanufacturing. Crit Rev Biotechnol 38(5):690–703. https://doi.org/10.1080/07388551.2017.1390728
Bradáčová K, Florea AS, Bar-Tal A, Minz D, Yermiyahu U, Shawahna R, Kraut-Cohen J, Zolti A, Erel R, Dietel K, Weinmann M (2019) Microbial consortia versus single-strain inoculants: an advantage in PGPM-assisted tomato production? Agronomy 9(2):105
Timmis K, Vos WMD, Ramos JL, Vlaeminck SE, Prieto A, Danchin A, Verstraete W, Lorenzo VD, Lee SY, Brüssow H, Singh BK (2017) The contribution of microbial biotechnology to sustainable development goals. Microbial biotechnology 10(5):984–987
Chu WL (2017) Strategies to enhance production of microalgal biomass and lipids for biofuel feedstock. Eur J Phycol 52(4):419–437. https://doi.org/10.1080/09670262.2017.1379100
Ramírez-Verduzco LF, Rodríguez-Rodríguez JE, del Rayo J-JA (2012) Predicting cetane number, kinematic viscosity, density and higher heating value of biodiesel from its fatty acid methyl ester composition. Fuel 91(1):102–111. https://doi.org/10.1016/j.fuel.2011.06.070
Bahafid W, Joutey NT, Asri M, Sayel H, Tirry N, El Ghachtouli N, Sayel NT (2017) Yeast biomass: an alternative for bioremediation of heavy metals. Yeast-Industrial Applications. https://doi.org/10.5772/intechopen.70559
Ueda H, Otsuka S, Senoo K (2009) Bacterial communities constructed in artificial consortia of bacteria and Chlorella vulgaris. Microbes Environ:1002020160. https://doi.org/10.1264/jsme2.ME09177
Ishika T, Moheimani NR, Bahri PA (2017) Sustainable saline microalgae co-cultivation for biofuel production: a critical review. Renew Sust Energ Rev 78:356–368. https://doi.org/10.1016/j.rser.2017.04.110
Arora M, Anil AC, Leliaert F, Delany J, Mesbahi E (2013) Tetraselmis indica (Chlorodendrophyceae, Chlorophyta), a new species isolated from salt pans in Goa, India. Eur J Phycol 48(1):61–78. https://doi.org/10.1080/09670262.2013.768357
Boonma S, Takarada T, Peerapornpisal Y, Pumas C, Chaiklangmuang S (2019) Semi-continuous cultivation of microalgal consortium using low CO2 concentration for large-scale biofuel production. J Biotech Res 10:19–28
Bogen C, Klassen V, Wichmann J, La Russa M, Doebbe A, Grundmann M, Uronen P, Kruse O, Mussgnug JH (2013) Identification of Monoraphidium contortum as a promising species for liquid biofuel production. Bioresour Technol 133:622–626. https://doi.org/10.1016/j.biortech.2013.01.164
Ramanan R, Kim BH, Cho DH, Oh HM, Kim HS (2016) Algae–bacteria interactions: evolution, ecology and emerging applications. Biotechnol Adv 34(1):14–29. https://doi.org/10.1016/j.biotechadv.2015.12.003
Zhao P, Yu X, Li J, Tang X, Huang Z (2014) Enhancing lipid productivity by co-cultivation of Chlorella sp. U4341 and Monoraphidium sp. FXY-10. J Biosci Bioeng 118(1):72–77
Asmare AM, Demessie BA, Murthy GS (2014) Investigation of Microalgae Co-Cultures for Nutrient Recovery and Algal Biomass Production from Dairy Manure. Appl Eng Agric 30(2):335–342
Sharma J, Kumar V, Kumar SS, Malyan SK, Mathimani T, Bishnoi NR, Pugazhendhi A (2020) Microalgal consortia for municipal wastewater treatment–Lipid augmentation and fatty acid profiling for biodiesel production. J Photoch Photobio B 202:111638
Ahmed Al Darmaki LG, Talebi S, Al-Rajhi S, Tahir Al-Barwani ZA (2012) Cultivation and characterization of microalgae for wastewater treatment. Proc World Congr Eng 1:4–7, London, U.K,
Sharma N, Dwivedi A (2017) Bioremediation dairy wastewater for nitrate reduction. World J Pharm Life Sci 3:375–384
Koreivienė J, Valčiukas R, Karosienė J, Baltrėnas P (2014) Testing of Chlorella/Scenedesmus microalgae consortia for remediation of wastewater, CO2 mitigation and algae biomass feasibility for lipid production. J Environ Eng Landsc 22(2):105–114. https://doi.org/10.3846/16486897.2013.911182
Pires JC, Alvim-Ferraz MC, Martins FG, Simões M (2013) Wastewater treatment to enhance the economic viability of microalgae culture. Environ Sci Pollut R 20(8):5096–5105. https://doi.org/10.1007/s11356-013-1791-x
Gopalakrishnan K, Roostaei J, Zhang Y (2018) Mixed culture of Chlorella sp. and wastewater wild algae for enhanced biomass and lipid accumulation in artificial wastewater medium. Front Environ Sci Eng 12(4):14. https://doi.org/10.1007/s11783-018-1075-2
Wilson MH, Shea A, Groppo J, Crofcheck C, Quiroz D, Quinn JC, Crocker M (2020) Algae-Based Beneficial Re-use of Carbon Emissions Using a Novel Photobioreactor: a Techno-Economic and Life Cycle Analysis. Bioenergy Res 8:1–1. https://doi.org/10.1007/s12155-020-10178-9
Fuentes JL, Garbayo I, Cuaresma M, Montero Z, González-del-Valle M, Vílchez C (2016) Impact of microalgae-bacteria interactions on the production of algal biomass and associated compounds. Mar Drugs 14(5):100. https://doi.org/10.3390/md14050100
Yao S, Lyu S, An Y, Lu J, Gjermansen C, Schramm A (2019) Microalgae–bacteria symbiosis in microalgal growth and biofuel production: a review. J Appl Microbiol 126(2):359–368. https://doi.org/10.1111/jam.14095
Sapp M, Schwaderer AS, Wiltshire KH, Hoppe HG, Gerdts G, Wichels A (2007) Species-specific bacterial communities in the phycosphere of microalgae? Microb Ecol 53(4):683–699. https://doi.org/10.1007/s00248-006-9162-5
Kazamia E, Czesnick H, Nguyen TT, Croft MT, Sherwood E, Sasso S, Hodson SJ, Warren MJ, Smith AG (2012) Mutualistic interactions between vitamin B12-dependent algae and heterotrophic bacteria exhibit regulation. Environ Microbial 14(6):1466–1476. https://doi.org/10.1111/j.1462-2920.2012.02733.x
Sun XM, Ren LJ, Zhao QY, Ji XJ, Huang H (2018) Microalgae for the production of lipid and carotenoids: a review with focus on stress regulation and adaptation. Biotechnol Biofuels 11(1):272. https://doi.org/10.1186/s13068-018-1275-9
Nef C, Jung S, Mairet F, Kaas R, Grizeau D, Garnier M (2019) How haptophytes microalgae mitigate vitamin B 12 limitation. Sci Rep 9(1):8417. https://doi.org/10.1038/s41598-019-44797-w
Durham BP, Sharma S, Luo H, Smith CB, Amin SA, Bender SJ, Dearth SP, Van Mooy BA, Campagna SR, Kujawinski EB, Armbrust EV (2015) Cryptic carbon and sulfur cycling between surface ocean plankton. Proc Natl Acad Sci 112(2):453–457. https://doi.org/10.1073/pnas.1413137112
Molina E, Fernández J, Acién FG, Chisti Y (2001) Tubular photobioreactor design for algal cultures. J Biotechnol 92(2):113–131. https://doi.org/10.1016/S0168-1656(01)00353-4
Bilanovic D, Holland M, Starosvetsky J, Armon R (2016) Co-cultivation of microalgae and nitrifiers for higher biomass production and better carbon capture. Bioresour Technol 220:282–288. https://doi.org/10.1016/j.biortech.2016.08.083
Acar C, Dincer I (2014) Comparative assessment of hydrogen production methods from renewable and non-renewable sources. Int J Hydrogen Energ 39(1):1–2. https://doi.org/10.1016/j.ijhydene.2013.10.060
MiuRA Y, Saitoh C, Matsuoka S, Miyamoto K (1992) Stably sustained hydrogen production with high molar yield through a combination of a marine green alga and a photosynthetic bacterium. Biosci Biotech Biochem 56(5):751–754. https://doi.org/10.1271/bbb.56.751
Kawaguchi H, Hashimoto K, Hirata K, Miyamoto K (2001) H2 production from algal biomass by a mixed culture of Rhodobium marinum A-501 and Lactobacillus amylovorus. J Biosci Bioeng 91(3):277–282. https://doi.org/10.1016/S1389-1723(01)80134-1
Contreras-Angulo JR, Mata TM, Cuellar-Bermudez SP, Caetano NS, Chandra R, Garcia-Perez JS, Muylaert K, Parra-Saldivar R (2019) Symbiotic co-culture of Scenedesmus sp. and Azospirillum brasilense on N-deficient media with biomass production for biofuels. Sustainability 11(3):707
Tsolcha ON, Tekerlekopoulou AG, Akratos CS, Antonopoulou G, Aggelis G, Genitsaris S, Moustaka-Gouni M, Vayenas DV (2018) A Leptolyngbya-based microbial consortium for agro-industrial wastewaters treatment and biodiesel production. Env Sci Pollut Res 25(18):17957–17966. https://doi.org/10.1007/s11356-018-1989-z
Wrede D, Taha M, Miranda AF, Kadali K, Stevenson T, Ball AS, Mouradov A (2014) Co-cultivation of fungal and microalgal cells as an efficient system for harvesting microalgal cells, lipid production and wastewater treatment. PloS one 9(11):e113497. https://doi.org/10.1371/journal.pone.0113497
Muradov N, Taha M, Miranda AF, Wrede D, Kadali K, Gujar A, Stevenson T, Ball AS, Mouradov A (2015) Fungal-assisted algal flocculation: application in wastewater treatment and biofuel production. Biotechnol Biofuels 8(1):24. https://doi.org/10.1186/s13068-015-0210-6
Wang R, Tian Y, Xue S, Zhang D, Zhang Q, Wu X, Kong D, Cong W (2016) Enhanced microalgal biomass and lipid production via co-culture of Scenedesmus obliquus and Candida tropicalis in an autotrophic system. J Chem Technol Biot 91(5):1387–1396. https://doi.org/10.1002/jctb.4735
Liu L, Chen J, Lim PE, Wei D (2018) Dual-species cultivation of microalgae and yeast for enhanced biomass and microbial lipid production. J Appl Phycol 30(6):2997–3007. https://doi.org/10.1007/s10811-018-1526-y
Rajapitamahuni S, Bachani P, Sardar RK, Mishra S (2019) Co-cultivation of siderophore-producing bacteria Idiomarina loihiensis RS14 with Chlorella variabilis ATCC 12198, evaluation of micro-algal growth, lipid, and protein content under iron starvation. J Appl Phycol 31(1):29–39
Berthold DE, Shetty KG, Jayachandran K, Laughinghouse HD IV, Gantar M (2019) Enhancing algal biomass and lipid production through bacterial co-culture. Biomass bioenergy 122:280–289. https://doi.org/10.1016/j.biombioe.2019.01.033
Ahmad JS, Cai W, Zhao Z, Zhang Z, Shimizu K, Lei Z, Lee DJ (2017) Stability of algal-bacterial granules in continuous-flow reactors to treat varying strength domestic wastewater. Bioresour Technol 244:225–233. https://doi.org/10.1016/j.biortech.2017.07.134
Markou G, Vandamme D, Muylaert K (2014) Microalgal and cyanobacterial cultivation: the supply of nutrients. Water Res 65:186–202. https://doi.org/10.1016/j.watres.2014.07.025
Lin C, Cao P, Xu X, Ye B (2019) Algal-bacterial symbiosis system treating high-load printing and dyeing wastewater in continuous-flow reactors under natural light. Water 11(3):469. https://doi.org/10.3390/w11030469
Xu M, Xue Z, Sun S, Zhao C, Liu J, Liu J, Zhao Y (2020) Co-culturing microalgae with endophytic bacteria increases nutrient removal efficiency for biogas purification. Bioresour Technol 314:123766. https://doi.org/10.1016/j.biortech.2020.123766
Yuan X, Xiao S, Taylor TN (2005) Lichen-like symbiosis 600 million years ago. Science 308(5724):1017–1020. https://doi.org/10.1126/science.1111347
Zhou W, Cheng Y, Li Y, Wan Y, Liu Y, Lin X, Ruan R (2012) Novel fungal pelletization-assisted technology for algae harvesting and wastewater treatment. Appl Biochem Biotechnol 167(2):214–228. https://doi.org/10.1007/s12010-012-9667-y
Veiter L, Rajamanickam V, Herwig C (2018) The filamentous fungal pellet—relationship between morphology and productivity. Appl Microbiol Biot 102(7):2997–3006. https://doi.org/10.1007/s00253-018-8818-7
Gultom SO, Zamalloa C, Hu B (2014) Microalgae harvest through fungal pelletization—co-culture of Chlorella vulgaris and Aspergillus niger. Energies 7(7):4417–4429. https://doi.org/10.3390/en7074417
Xia C, Zhang J, Zhang W, Hu B (2011) A new cultivation method for microbial oil production: cell pelletization and lipid accumulation by Mucor circinelloides. Biotechnol biofuels 4(1):1-0. doi:https://doi.org/10.1186/1754-6834-4-15
Dash A, Banerjee R (2017) Enhanced biodiesel production through phyco-myco co-cultivation of Chlorella minutissima and Aspergillus awamori: an integrated approach. Bioresour Technol 238:502–509. https://doi.org/10.1016/j.biortech.2017.04.039
Toscano L, Ogden KL, Ogden G, Cervantes L, Steichen SA, Brown C, Samaniego BY, Brown JK (2018) Harvesting the Microalga Chlorella sorokiniana by Fungal-Assisted Pelletization. J Biobased Mater Bio 12(6):493–505. https://doi.org/10.1166/jbmb.2018.1798
Bhatnagar VS, Bandyopadhyay P, Rajacharya GH, Sarkar S, Poluri KM, Kumar S (2019) Amelioration of biomass and lipid in marine alga by an endophytic fungus Piriformospora indica. Biotechnol Biofuels 12(1):176. https://doi.org/10.1186/s13068-019-1516-6
Du ZY, Alvaro J, Hyden B, Zienkiewicz K, Benning N, Zienkiewicz A, Bonito G, Benning C (2018) Enhancing oil production and harvest by combining the marine alga Nannochloropsis oceanica and the oleaginous fungus Mortierella elongata. Biotechnol Biofuels 11(1):1-6. doi:https://doi.org/10.1186/s13068-018-1172-2.
Yang L, Li H, Wang Q (2019) A novel one-step method for oil-rich biomass production and harvesting by co-cultivating microalgae with filamentous fungi in molasses wastewater. Bioresour Technol 275:35–43
Naidoo RK, Simpson ZF, Oosthuizen JR, Bauer FF (2019) Nutrient exchange of carbon and nitrogen promotes the formation of stable mutualisms between Chlorella sorokiniana and Saccharomyces cerevisiae under engineered synthetic growth conditions. Front Microbial 10:609
Simpson ZF (2018) Engineered yeast and microalgae mutualisms: Synthetic ecology applied to species isolated from winery wastewater (Master’s dissertation, Stellenbosch: Stellenbosch University).
Chi Z, Zheng Y, Jiang A, Chen S (2011) Lipid production by culturing oleaginous yeast and algae with food waste and municipal wastewater in an integrated process. Appl Biochem Biotechnol 165(2):442–453. https://doi.org/10.1007/s12010-011-9263-6
De-Bashan LE, Moreno M, Hernandez JP, Bashan Y (2002) Removal of ammonium and phosphorus ions from synthetic wastewater by the microalgae Chlorella vulgaris coimmobilized in alginate beads with the microalgae growth-promoting bacterium Azospirillum brasilense. Water Res 36(12):2941–2948. https://doi.org/10.1016/S0043-1354(01)00522-X
Mujtaba G, Rizwan M, Lee K (2015) Simultaneous removal of inorganic nutrients and organic carbon by symbiotic co-culture of Chlorella vulgaris and Pseudomonas putida. Biotechnol Bioprocess Eng 20(6):1114–1122. https://doi.org/10.1007/s12257-015-0421-5
Mujtaba G, Lee K (2016) Advanced treatment of wastewater using symbiotic co-culture of microalgae and bacteria. Appl Chem Eng 27(1):1–9. https://doi.org/10.14478/ace.2016.1002
González C, Marciniak J, Villaverde S, Leon C, Garcia PA, Munoz R (2008) Efficient nutrient removal from swine manure in a tubular biofilm photo-bioreactor using algae-bacteria consortia. Water Sci Technol 58(1):95–102. https://doi.org/10.2166/wst.2008.655
de Godos I, Vargas VA, Blanco S, González MC, Soto R, García-Encina PA, Becares E, Muñoz R (2010) A comparative evaluation of microalgae for the degradation of piggery wastewater under photosynthetic oxygenation. Bioresour Technol 101(14):5150–5158. https://doi.org/10.1016/j.biortech.2010.02.010
Li T, Li CT, Butler K, Hays SG, Guarnieri MT, Oyler GA, Betenbaugh MJ (2017) Mimicking lichens: incorporation of yeast strains together with sucrose-secreting cyanobacteria improves survival, growth, ROS removal, and lipid production in a stable mutualistic co-culture production platform. Biotechnol Biofuels 10(1):1–1. https://doi.org/10.1186/s13068-017-0736-x
Arora N, Patel A, Mehtani J, Pruthi PA, Pruthi V, Poluri KM (2019) Co-culturing of oleaginous microalgae and yeast: paradigm shift towards enhanced lipid productivity. Environ Sci Pollut Res:1–22. https://doi.org/10.1007/s11356-019-05138-6
Suastes-Rivas JK, Hernández-Altamirano R, Mena-Cervantes VY, Chairez I (2020) Simultaneous Optimization of Biomass and Metabolite Production by a Microalgae-Yeast Co-culture Under Inorganic Micronutrients. Bioenergy Res 17:1–2. https://doi.org/10.1007/s12155-020-10116-9
Qin L, Liu L, Zeng AP, Wei D (2017) From low-cost substrates to single cell oils synthesized by oleaginous yeasts. Bioresour Technol 245:1507–1519. https://doi.org/10.1016/j.biortech.2017.05.163
Xue F, Miao J, Zhang X, Tan T (2010) A new strategy for lipid production by mix cultivation of Spirulina platensis and Rhodotorula glutinis. Appl Biochem Biotechnol 160(2):498–503. https://doi.org/10.1007/s12010-008-8376-z
Zhang K, Zheng J, Xue D, Ren D, Lu J (2017) Effect of photoautotrophic and heteroautotrophic conditions on growth and lipid production in Chlorella vulgaris cultured in industrial wastewater with the yeast Rhodotorula glutinis. J Appl Phycol 29(6):2783–2788. https://doi.org/10.1007/s10811-017-1168-5
Papone T, Kookkhunthod S, Leesing R (2012) Microbial oil production by monoculture and mixed cultures of microalgae and oleaginous yeasts using sugarcane juice as substrate. World Acad Sci Eng Technol 64:1127–1131. https://doi.org/10.5281/zenodo.1332532
Kitcha S, Cheirsilp B (2014) Enhanced lipid production by co-cultivation and co-encapsulation of oleaginous yeast Trichosporonoides spathulata with microalgae in alginate gel beads. Appl Biochem Biotechnol 173(2):522–534. https://doi.org/10.1007/s12010-014-0859-5
Tian YT, Wang X, Cui YH, Wang SK (2020) A symbiotic yeast to enhance heterotrophic and mixotrophic cultivation of Chlorella pyrenoidosa using sucrose as the carbon source. Bioproc Biosyst Eng 43(12):2243–2252. https://doi.org/10.1007/s00449-020-02409-2
Zhou W, Wang W, Li Y, Zhang Y (2013) Lipid production by Rhodosporidium toruloides Y2 in bioethanol wastewater and evaluation of biomass energetic yield. Bioresour Technol 127:435–440
Iasimone F, Zuccaro G, D'Oriano V, Franci G, Galdiero M, Pirozzi D, De Felice V, Pirozzi F (2018) Combined yeast and microalgal cultivation in a pilot-scale raceway pond for urban wastewater treatment and potential biodiesel production. Water Sci Technol 77(4):1062–1071. https://doi.org/10.2166/wst.2017.620
Waghmode TR, Kurade MB, Govindwar SP (2011) Time dependent degradation of mixture of structurally different azo and non azo dyes by using Galactomyces geotrichum MTCC 1360. Int Biodeter Biodegr 65(3):479–486. https://doi.org/10.1016/j.ibiod.2011.01.010
Li H, Zhong Y, Lu Q, Zhang X, Wang Q, Liu H, Diao Z, Yao C, Liu H (2019) Co-cultivation of Rhodotorula glutinis and Chlorella pyrenoidosa to improve nutrient removal and protein content by their synergistic relationship. RSC Adv 9(25):14331–14342. https://doi.org/10.1039/c9ra01884k
Bahafid W, Tahri Joutey N, Sayel H, Boularab I, El Ghachtouli N (2013) Bioaugmentation of chromium-polluted soil microcosms with Candida tropicalis diminishes phytoavailable chromium. J Appl Microbial 115(3):727–734. https://doi.org/10.1111/jam.12282
Saad MG, Dosoky NS, Zoromba MS, Shafik HM (2019) Algal biofuels: Current status and key challenges. Energies 12(10):1920. https://doi.org/10.3390/en12101920
Doe US (2010) Office of Energy Efficiency and Renewable Energy. National algal biofuels technology roadmap
Fasaei F, Bitter JH, Slegers PM, Van Boxtel AJ (2018) Techno-economic evaluation of microalgae harvesting and dewatering systems. Algal Res 31:347–362. https://doi.org/10.1016/j.algal.2017.11.038
Geissler CH, Mulligan ML, Zmola ZE, Ray S, Morgan JA, Garner AL (2020) Electric Pulse Pretreatment for Enhanced Lipid Recovery from Chlorella protothecoides. Bioenergy Res 13(2):499–506. https://doi.org/10.1007/s12155-019-10064-z
Ranjith Kumar R, Hanumantha Rao P, Arumugam M (2015) Lipid extraction methods from microalgae: a comprehensive review. Front Energy Res 2:61. https://doi.org/10.3389/fenrg.2014.00061
Chung JN (2013) Grand challenges in bioenergy and biofuel research: engineering and technology development, environmental impact, and sustainability. Front Energy Res 1:4. https://doi.org/10.3389/fenrg.2013.00004
Amin M, Chetpattananondh P (2019) Enhanced Lipid Recovery from Marine Chlorella sp. by Ultrasonication with an Integrated Process Approach for Wet and Dry Biomass. Bioenergy Res 12(3):665–679. https://doi.org/10.1007/s12155-019-09986-5
Sanchez AS, Matos AP (2018) Desalination concentrate management and valorization methods. In: Sustainable Desalination Handbook
Srinivasana M, Venkatesanb M, Arumugamc V, Natesand G, Saravananb N, Murugesane S, Ramachandranb S, Ayyasamyf R, Pugazhendhig A (2019) Green synthesis and characterization of titanium dioxide nanoparticles (TiO2 NPs) using Sesbania grandiflora and evaluation of toxicity in zebrafish embryos. Process Biochem 80:197–202. https://doi.org/10.1016/j.procbio.2019.02.010
Shankar PD, Shobana S, Karuppusamy I, Pugazhendhi A, Ramkumar VS, Arvindnarayan S, Kumar G (2016) A review on the biosynthesis of metallic nanoparticles (gold and silver) using bio-components of microalgae: formation mechanism and applications. Enzyme Microb Technol 95:28–44. https://doi.org/10.1016/j.enzmictec.2016.10.015
Hariharana D, Thangamuniyandib P, Christyc AJ, Vasantharajad R, Selvakumare P, Sagadevanf S, Pugazhendhig A, Nehrua LC (2020) Enhanced photocatalysis and anticancer activity of green hydrothermal synthesized Ag@TiO2 nanoparticles. J Photoch Photobio B 202:111636
Gen Q, Wang Q, Chi ZM (2014) Direct conversion of cassava starch into single cell oil by co-cultures of the oleaginous yeast Rhodosporidium toruloides and immobilized amylases-producing yeast Saccharomycopsis fibuligera. Renew Energy 62:522–526. https://doi.org/10.1016/j.renene.2013.08.016
DellaGreca M, Zarrelli A, Fergola P, Cerasuolo M, Pollio A, Pinto G (2010) Fatty acids released by Chlorella vulgaris and their role in interference with Pseudokirchneriella subcapitata: experiments and modelling. J Chem Ecol 36(3):339–349. https://doi.org/10.1007/s10886-010-9753-y
Coleman RA, Lewin TM, Van Horn CG, Gonzalez-Baró MR (2002) Do long-chain acyl-CoA synthetases regulate fatty acid entry into synthetic versus degradative pathways? J Nutr 132(8):2123–2126. https://doi.org/10.1093/jn/132.8.2123
Salama ES, Hwang JH, El-Dalatony MM, Kurade MB, Kabra AN, Abou-Shanab RA, Kim KH, Yang IS, Govindwar SP, Kim S, Jeon BH (2018) Enhancement of microalgal growth and biocomponent-based transformations for improved biofuel recovery: A review. Bioresour Technol 258:365–375. https://doi.org/10.1016/j.biortech.2018.02.006
Grant MA, Kazamia E, Cicuta P, Smith AG (2014) Direct exchange of vitamin B-12 is demonstrated by modelling the growth dynamics of algal–bacterial cocultures. Int Soci Microb Ecol 8(7):1418–1427
Tan ZQ, Leow HY, Lee DC, Karisnan K, Song AA, Mai CW, Yap WS, Lim SH, Lai KS (2019) Co-culture Systems for the production of secondary metabolites: Current and future prospects. Open Biotechnol J 13:18–26. https://doi.org/10.2174/1874070701913010018
Wu N (2018) Techno-economic analysis of biofuels production (Unpublished doctoral dissertation). University of Florida, Florida, USA
Kang S, Heo S, Lee JH (2018) Techno-economic Analysis of Microalgae-Based Lipid Production: Considering Influences of Microalgal Species. Ind Eng Chem 58(2):944–955. https://doi.org/10.1021/acs.iecr.8b03999
Kang Z, Kim BH, Ramanan R, Choi JE, Yang JW, Oh HM, Kim HS (2015) A cost analysis of microalgal biomass and biodiesel production in open raceways treating municipal wastewater and under optimum light wavelength. J Microbiol Biotechnol 25(1):109–118. https://doi.org/10.4014/jmb.1409.09019
Slegers PM, Olivieri G, Breitmayer E, Sijtsma L, Eppink MH, Wijffels RH, Reith JH (2020) Design of Value Chains for Microalgal Biorefinery at Industrial Scale: Process Integration and Techno-Economic Analysis. Front Bioeng Biotechnol 8:1048. https://doi.org/10.3389/fbioe.2020.550758
Singh B, Bauddh K, Bux F (2015) Algae and environmental sustainability. Springer, India
Funding
PKD and JR are financially supported by the Ministry of Human Resources and Development (MHRD), Government of India. SK is supported by a seed grant project from IIT (BHU) Varanasi for the work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Das, P.K., Rani, J., Rawat, S. et al. Microalgal Co-cultivation for Biofuel Production and Bioremediation: Current Status and Benefits. Bioenerg. Res. 15, 1–26 (2022). https://doi.org/10.1007/s12155-021-10254-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12155-021-10254-8