Abstract
Purpose
This study aimed to investigate the incidence of rim uptake (RU) or multifocal uptake (MU) by invasive breast cancers on a ring-type dedicated breast positron emission tomography (dbPET) scanner compared with whole-body PET (wbPET) scanner imaging and to correlate uptake patterns with pathological features and prognosis.
Methods
Between 2009 and 2011, 76 lesions in 74 patients with primary invasive breast cancers were included. Each patient underwent dbPET and wbPET scanning on the same day after administration of 18F-fluorodeoxyglucose (FDG). The images were evaluated to identify specific uptake patterns (RU and MU). Their association with pathological characteristics and prognosis was analyzed.
Results
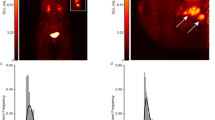
On dbPET, RU and MU patterns were observed in 18 lesions (24%) and 28 lesions (37%), respectively. On wbPET, RU and MU patterns were observed in six lesions (8%) and 17 lesions (22%), respectively. Lesions with RU on dbPET were of higher grade than lesions without RU (P = 0.024) and a higher Ki-67 index (mean; 31% vs. 18%, P = 0.015). They tended to be triple-negative (33% vs. 12%, P = 0.046) and less likely to be luminal A subtype (17% vs. 47%, P = 0.020). On wbPET, however, no significant differences in these markers were seen between RU and non-RU. The MU pattern did not correlate with pathological characteristics in either scanner. Lesions with RU or MU were not significantly associated with disease-free survival.
Conclusions
DbPET can identify detailed FDG distribution patterns of breast cancer better than wbPET. Breast cancer with RU on dbPET was associated with higher grade and triple-negative subtype.






Similar content being viewed by others
References
Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology. 1991;19:403–10.
Sørlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA. 2001;98:10869–74.
Prat A, Perou CM. Deconstructing the molecular portraits of breast cancer. Mol Oncol. 2011;5:5–23.
Goldhirsch A, Wood WC, Coates AS, Gelber RD, Thürlimann B, Senn HJ, et al. Strategies for subtypes—dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol. 2011;22:1736–47.
Romero Q, Bendahl PO, Klintman M, Loman N, Ingvar C, Rydén L, et al. Ki67 proliferation in core biopsies versus surgical samples—a model for neo-adjuvant breast cancer studies. BMC Cancer. 2011;11:341.
Sorlie T, Tibshirani R, Parker J, Hastie T, Marron JS, Nobel A, et al. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci USA. 2003;100:8418–23.
Groheux D, Espie M, Giacchetti S, Hindie E. Performance of FDG PET/CT in the clinical management of breast cancer. Radiology. 2013;266:388–405.
Bowen SL, Wu Y, Chaudhari AJ, Fu L, Packard NJ, Burkett GW, et al. Initial characterization of a dedicated breast PET/CT scanner during human imaging. J Nucl Med. 2009;50:1401–8.
Koolen BB, Vogel WV, Vrancken Peeters MJ, Loo CE, Rutgers EJ, Valdes Olmos RA. Molecular imaging in breast cancer: from whole-body PET/CT to dedicated breast PET. J Oncol. 2012;2012:438647.
Iima M, Nakamoto Y, Kanao S, Sugie T, Ueno T, Kawada M, et al. Clinical performance of two dedicated PET scanners for breast imaging: initial evaluation. J Nucl Med. 2012;53:1534–42.
Koyasu S, Nakamoto Y, Kikuchi M, Suzuki K, Hayashida K, Itoh K, et al. Prognostic value of pretreatment 18F-FDG PET/CT parameters including visual evaluation in patients with head and neck squamous cell carcinoma. AJR Am J Roentgenol. 2014;202:851–8.
Miyake KK, Nakamoto Y, Mikami Y, Tanaka S, Higashi T, Tadamura E, et al. The predictive value of preoperative (18)F-fluorodeoxyglucose PET for postoperative recurrence in patients with localized primary gastrointestinal stromal tumour. Eur Radiol. 2016;26:4664–74.
Miyake KK, Matsumoto K, Inoue M, Nakamoto Y, Kanao S, Oishi T, et al. Performance evaluation of a new dedicated breast PET scanner using NEMA NU4-2008 standards. J Nucl Med. 2014;55:1198–203.
Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–74.
Masumoto N, Kadoya T, Sasada S, Emi A, Arihiro K, Okada M. Intratumoral heterogeneity on dedicated breast positron emission tomography predicts malignancy grade of breast cancer. Breast Cancer Res Treat. 2018;171:315–23.
Broome DR. Nephrogenic systemic fibrosis associated with gadolinium based contrast agents: a summary of the medical literature reporting. Eur J Radiol. 2008;66(2):230–4.
Morris EA, Comstock CE, Lee CH, et al. ACR BI-RADS® magnetic resonance imaging. In: ACR BI-RADS® Atlas, breast imaging reporting and data system. Reston: American College of Radiology; 2013
Jimenez RE, Wallis T, Visscher DW. Centrally necrotizing carcinomas of the breast: a distinct histologic subtype with aggressive clinical behavior. Am J Surg Pathol. 2001;25:331–7.
Yu L, Yang W, Cai X, Shi D, Fan Y, Lu H. Centrally necrotizing carcinoma of the breast: clinicopathological analysis of 33 cases indicating its basal-like phenotype and poor prognosis. Histopathology. 2010;57:193–201.
Hasebe T, Tsuda H, Hirohashi S, Shimosato Y, Iwai M, Imoto S, et al. Fibrotic focus in invasive ductal carcinoma: an indicator of high tumor aggressiveness. Jpn J Cancer Res. 1996;87:385–94.
Van den Eynden GG, Colpaert CG, Couvelard A, Pezzella F, Dirix LY, Vermeulen PB, et al. A fibrotic focus is a prognostic factor and a surrogate marker for hypoxia and (lymph)angiogenesis in breast cancer: review of the literature and proposal on the criteria of evaluation. Histopathology. 2007;51:440–51.
Ahmed S, Sami A, Xiang J. HER2-directed therapy: current treatment options for HER2-positive breast cancer. Breast Cancer. 2015;22:101–16.
Petrelli F, Coinu A, Borgonovo K, Cabiddu M, Ghilardi M, Lonati V, et al. The value of platinum agents as neoadjuvant chemotherapy in triple-negative breast cancers: a systematic review and meta-analysis. Breast Cancer Res Treat. 2014;144:223–32.
Acknowledgements
The authors would like to thank Dr. Mami Iima from Department of Diagnostic Imaging and Nuclear Medicine of Kyoto University Graduate School of Medicine for the excellent and knowledgeable support. We also thank Dr. Libby Cone from Edanz Group (www.edanzediting.com) for editing a draft of this manuscript.
Funding
This work was supported by Shimadzu Co., Kyoto, Japan, and a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan (C:22591329).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This work was supported by Shimadzu Co., Kyoto, Japan, and a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan (C:22591329).
Rights and permissions
About this article
Cite this article
Sakaguchi, R., Kataoka, M., Kanao, S. et al. Distribution pattern of FDG uptake using ring-type dedicated breast PET in comparison to whole-body PET/CT scanning in invasive breast cancer. Ann Nucl Med 33, 570–578 (2019). https://doi.org/10.1007/s12149-019-01364-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12149-019-01364-7