Abstract
G3BP1 exists as a helicase and one of the core components in stress granules, which are associated with a variety of neurodegenerative diseases. Its RNA recognition motif (RRM) domain performs the paramount function of binding mRNA. Here we report the resonance assignment of human G3BP1 RRM domain to understand its structure–function relationship.
Similar content being viewed by others
Biological context
Stress granules occur in vivo when eukaryotic cells are stimulated by oxidation, arsenite, ultraviolet rays, viruses, etc. (Courchaine et al. 2016; Ryan et al. 2018). They are an aggregate of RNAs and their binding proteins (Nott et al. 2015). A variety of neurodegenerative diseases and cancers are associated with eukaryotic stress granules (Conicella et al. 2016; Ambadipudi et al. 2019). Also stress granules isolate viral RNA in antiviral events. GTPase-activating protein (SH3 domain)-binding protein 1 (G3BP1) has been reported as an essential protein in stress granules in response to arsenite (Yang et al. 2020). As an RNA endonuclease in both nucleus and cytoplasm, G3BP1 also plays an important role in immune response and stress response. G3BP1 contains a nuclear transport factor 2 (NTF2) region, an RRM region and several intrinsically disordered regions. It intertwines with mRNA and recruits many downstream proteins to form stress granules together.
G3BP1 is associated with severe human diseases. As Alzheimer's disease progresses, G3BP gradually accumulates in neurons (Vanderweyde et al. 2012). In amyotrophic lateral sclerosis (ALS) or frontotemporal dementia (FTD), the stability of G3BP1 mRNA is impaired due to nuclear depletion of TDP-43, so the stress granules in motor neurons are abnormal (Sidibe et al. 2021). Recently, it has been reported that G3BP1 plays an important role in the treatment of SARS-CoV-2 for its function in mediating the interaction of G3BPs with nucleocapsid N protein. By combining with the N protein, G3BP1 achieves the purpose of blocking RNA replication (Luo et al. 2021).
Up to now, it's already clear many diseases are directly or indirectly related to dysfunctional G3BP1, and it is of great significance to study its structure. Here we report the backbone assignment of of G3BP1 RRM domain, with > 99% of residues in the RRM domain being assigned unambiguously.
Methods and experiments
The polypeptide segment used in this task was residue 327–424 of the RRM domain in human G3BP1 protein. DNA segment encoding that polypeptide was acquired from embryonic brain cDNA library by PCR (the 5′-end primer was 5′-GGAATTCCATATGGACATTGAACCCCGAAG-3′, the 3′-end primer was 5′-CGACGTCGACTTATCGTCGGTCGCCTTCCC-3′). The DNA segment was cloned into pET28A vector between Nde1 and Sal1 restriction enzyme cutting site, adjacent to a His-tag. The recombinant plasmid was transformed into BL21 Escherichia coli strain, which was cultured in LR medium which contained 15 N-labeled NH4Cl (0.625 g/L) and 13C-labeled glucose (5 g/L) as sole nitrogen and carbon source Protein expression was induced by 0.4 mM isopropyl-β-D-thiogalacto-pyranoside (IPTG) at 310 K. After 5 h, cells containing the target protein were collected in a refrigerated centrifuge at 5000 rpm, resuspended with binding buffer (20 mM NaH2PO4, 2 M NaCl, pH 6.5), cracked in a high-pressure homogenizer, and centrifuged at 13,000 rpm to acquire the liquid supernatant, then it was added to a balanced Ni–NTA His binding resin column. The His-tag target protein was firstly washed with the washing buffer (20 mM NaH2PO4, 2 M NaCl, 20–50 mM imidazole, pH 6.5), then eluted with 7 mL elution buffer (20 mM NaH2PO4, 2 M NaCl, 500 mM imidazole, pH 6.5). Next, the target protein was put through a HiLoad superdex 75 column (GE), collected in low salt buffer (20 mM NaH2PO4, 150 mM NaCl, pH 6.5) and then concentrated to 2 mM.
3D NMR spectra including HN(CO)CA, HNCA, HACA(CO)NH, HACANH, HN(CO)CACB, HNCACB, HNCO and 1H-15 N HSQC were all acquired at 298 K on an Agilent 700 MHz spectrometer. They all contributed to backbone chemical shift assignments and dihedral information. Specifically speaking, HN(CO)CA and HNCA measured the chemical shifts of CA, N and NH, HN(CO)CACB and HNCACB measured the chemical shifts of CB, N and NH, HACA(CO)NH and HACANH measured the chemical shifts of HA, N and NH. Software NMRpipe, SPARKY and CARA were used in spectra process and data acquisition.
Extent of assignment and data deposition
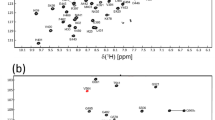
We assigned most of backbone resonances of the human G3BP1 RRM domain, including 100% of 1HN, 100% of 15 N, 100% of 13Cα, 98.9% of 13Cβ, 98.9% of 1HA, and 82.6% of 13CO chemical shifts. The backbone data was obtained from HN(CO)CA, HNCA, HN(CO)CACB, HNCACB, HACA(CO)NH, HACANH spectra. We showed the labeled 1H-15 N HSQC spectrum for G3BP1 RRM domain in Fig. 1, while the residues in His-tag were not assigned in this figure. The chemical shifts for prolines were also assigned, including those of Cα, Cβ for P330, P337, P348, P378, P390, P399 with the values (ppm): 62.809, 31.25, 62.051, 31.251, 61.581, 31.251, 63.061, 31.505, 65.083, 29.284, 61.184, 33.663, they were all within the normal range.
By using the consensus chemical shift index from all spectra mentioned above as well as HNCO, we found that the G3BP1 RRM domain contains two α-helixes and five β-strands. This result is basically consistent with the prediction result of alphafold (Fig. 2).
The chemical shift data have been deposited in the BioMagResBank (http://www.bmrb.wisc.edu) under accession number 51133.
Data availability
Some or all data, models, or code that support the findings of this manuscript are available from the corresponding author upon reasonable request.
References
Ambadipudi S et al (2019) Residue-specific identification of phase separation hot spots of Alzheimer’s-related protein tau. Chem Sci 10(26):6503–6507
Conicella AE et al (2016) ALS mutations disrupt phase separation mediated by alpha-helical structure in the TDP-43 low-complexity C-terminal domain. Structure 24(9):1537–1549
Courchaine EM, Lu A, Neugebauer KM (2016) Droplet organelles? EMBO J 35(15):1603–1612
Luo LL et al (2021) SARS-CoV-2 nucleocapsid protein phase separates with G3BPs to disassemble stress granules and facilitate viral production. Science Bulletin 66(12):1194–1204
Nott TJ et al (2015) Phase transition of a disordered nuage protein generates environmentally responsive membraneless organelles. Mol Cell 57(5):936–947
Ryan VH et al (2018) Mechanistic view of hnRNPA2 low-complexity domain structure, interactions, and phase separation altered by mutation and arginine methylation. Mol Cell. https://doi.org/10.1016/j.molcel.2017.12.022
Sidibe H et al (2021) TDP-43 stabilizes G3BP1 mRNA: relevance to amyotrophic lateral sclerosis/frontotemporal dementia. Brain. https://doi.org/10.1093/brain/awab217
Vanderweyde T et al (2012) Contrasting pathology of the stress granule proteins TIA-1 and G3BP in tauopathies. J Neurosci 32(24):8270–8283
Yang PG et al (2020) G3BP1 is a tunable switch that triggers phase separation to assemble stress granules. Cell 181(2):325
Acknowledgements
We thank F. Delaglio and A. Bax for providing NMRPipe and NMRDraw software. We thank Goddard and Kneller for providing SPARKY software. We also thank Fred Damberger and Rochus Keller for providing CARA software. This work was supported by the National Natural Science Foundation of China (32071220).
Funding
The Funder was funded by National Natural Science Foundation of China, Grant No (32071220).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
We declare that we do not have any commercial or associative interest that represents a conflict of interest in connection with the manuscript submitted.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhang, H., Tu, X. & Zhang, J. 1H, 13C and 15N resonance assignments of stress granule key component G3BP1 RRM domain. Biomol NMR Assign 16, 109–111 (2022). https://doi.org/10.1007/s12104-022-10067-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12104-022-10067-6