Abstract
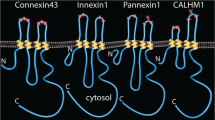
Connexin37 (Cx37) is a gap junction protein involved in cell-to-cell communication in the vasculature and other tissues. Cx37 suppresses proliferation of vascular cells involved in tissue development and repair in vivo, as well as tumor cells. Global deletion of Cx37 in mice leads to enhanced vasculogenesis in development, as well as collateralgenesis and angiogenesis in response to injury, which together support improved tissue remodeling and recovery following ischemic injury. Here we report the 1H, 15N, and 13C resonance assignments for an important regulatory domain of Cx37, the carboxyl terminus (CT; C233-V333). The predicted secondary structure of the Cx37CT domain based on the chemical shifts is that of an intrinsically disordered protein. In the 1H–15N HSQC, N-terminal residues S254-Y259 displayed a second weaker peak and residues E261-Y266 had significant line broadening. These residues are flanked by prolines (P250, P258, P260, and P268), suggesting proline cis–trans isomerization. Overall, these assignments will be useful for identifying the binding sites for intra- and inter-molecular interactions that affect Cx37 channel activity.


Similar content being viewed by others
References
Anumonwo JM, Taffet SM, Gu H, Chanson M, Moreno AP, Delmar M (2001) The carboxyl terminal domain regulates the unitary conductance and voltage dependence of connexin40 gap junction channels. Circ Res 88:666–673
Bouvier D, Kieken F, Sorgen PL (2007) (1)H, (13)C, and (15)N backbone resonance assignments of the carboxyl terminal domain of Connexin40. Biomol NMR Assign 1:155–157
Bouvier D, Kieken F, Kellezi A, Sorgen PL (2008) Structural changes in the carboxyl terminus of the gap junction protein connexin 40 caused by the interaction with c-Src and zonula occludens-1. Cell Commun Adhes 15:107–118
Brisset AC, Isakson BE, Kwak BR (2009) Connexins in vascular physiology and pathology. Antioxid Redox Signal 11:267–282
Delaglio F, Grzesiek S, Vuister GW, Zhu G, Pfeifer J, Bax A (1995) NMRPipe: a multidimensional spectral processing system based on UNIX pipes. J Biomol NMR 6:277–293
Dunker AK, Brown CJ, Lawson JD, Iakoucheva LM, Obradovic Z (2002) Intrinsic disorder and protein function. BioChemistry 41:6573–6582
Gilleron J, Carette D, Chevallier D, Segretain D, Pointis G (2012) Molecular connexin partner remodeling orchestrates connexin traffic: from physiology to pathophysiology. Crit Rev Biochem Mol Biol 47:407–423
Haefliger JA, Nicod P, Meda P (2004) Contribution of connexins to the function of the vascular wall. Cardiovasc Res 62:345–356
Harris AL (2001) Emerging issues of connexin channels: biophysics fills the gap. Q Rev Biophys 34:325–472
Herve J, Bourmeyster N, Sarrouilhe D, Duffy H (2007) Gap junctional complexes: from partners to functions. Prog Biophys Mol Biol 94:29–65
Johnson BA (2004) Using NMRView to visualize and analyze the NMR spectra of macromolecules. Methods Mol Biol 278:313–352
Kellezi A, Grosely R, Kieken F, Borgstahl GE, Sorgen PL (2008) Purification and reconstitution of the connexin43 carboxyl terminus attached to the 4th transmembrane domain in detergent micelles. Protein Expr Purif 59:215–222
Kieken F, Spagnol G, Su V, Lau AF, Sorgen PL (2010) NMR structure note: UBA domain of CIP75. J Biomol NMR 46:245–250
Kopanic JL, Sorgen PL (2013) Chemical shift assignments of the connexin45 carboxyl terminal domain: monomer and dimer conformations. Biomol NMR Assign 7:293–297
Laird DW (2010) The gap junction proteome and its relationship to disease. Trends Cell Biol 20:92–101
Lampe PD, Lau AF (2004) The effects of connexin phosphorylation on gap junctional communication. Int J Biochem Cell Biol 36:1171–1186
LeMaster DM, Richards FM (1988) NMR sequential assignment of Escherichia coli thioredoxin utilizing random fractional deuteriation. BioChemistry 27:142–150
Morel S (2014) Multiple roles of connexins in atherosclerosis- and restenosis-induced vascular remodelling. J Vasc Res 51:149–161
Moreno AP, Chanson M, Elenes S, Anumonwo J, Scerri I, Gu H, Taffet SM, Delmar M (2002) Role of the carboxyl terminal of connexin43 in transjunctional fast voltage gating. Circ Res 90:450–457
Morley GE, Taffet SM, Delmar M (1996) Intramolecular interactions mediate pH regulation of connexin43 channels. Biophys J 70:1294–1302
Nelson TK, Sorgen PL, Burt JM (2013) Carboxy terminus and pore-forming domain properties specific to Cx37 are necessary for Cx37-mediated suppression of insulinoma cell proliferation. Am J Physiol Cell Physiol 305:C1246–C1256
Revilla A, Castro C, Barrio LC (1999) Molecular dissection of transjunctional voltage dependence in the connexin-32 and connexin-43 junctions. Biophys J 77:1374–1383
Sorgen PL, Duffy HS, Sahoo P, Coombs W, Delmar M, Spray DC (2004a) Structural changes in the carboxyl terminus of the gap junction protein connexin43 indicates signaling between binding domains for c-Src and zonula occludens-1. J Biol Chem 279:54695–54701
Sorgen PL, Duffy HS, Spray DC, Delmar M (2004b) pH-dependent dimerization of the carboxyl terminal domain of Cx43. Biophys J 87:574–581
Stauch K, Kieken F, Sorgen P (2012) Characterization of the structure and intermolecular interactions between the connexin 32 carboxyl-terminal domain and the protein partners synapse-associated protein 97 and calmodulin. J Biol Chem 287:27771–27788
Thevenin AF, Kowal TJ, Fong JT, Kells RM, Fisher CG, Falk MM (2013). Proteins and mechanisms regulating gap-junction assembly, internalization, and degradation. Physiology 28:93–116
Vourtsis DJ, Chasapis CT, Pairas G, Bentrop D, Spyroulias GA (2014) NMR conformational properties of an Anthrax Lethal Factor domain studied by multiple amino acid-selective labeling. Biochem Biophys Res Commun 450:335–340
Wishart DS, Sykes BD (1994) The 13 C chemical-shift index: a simple method for the identification of protein secondary structure using 13 C chemical-shift data. J Biomol NMR 4:171–180
Acknowledgements
This work is funded by the United States Public Health Service Grants, GM072631, HL131712, CA036727, and GM103427. We would like to thank Ed Ezell, manager of the Nuclear Magnetic Resonance Laboratory at the University of Nebraska Medical Center, for his assistance with collection of the NMR data.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, H., Spagnol, G., Pontifex, T.K. et al. Chemical shift assignments of the connexin37 carboxyl terminal domain. Biomol NMR Assign 11, 137–141 (2017). https://doi.org/10.1007/s12104-017-9735-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12104-017-9735-x