Abstract
Aims
The primary standard treatment for classic Hodgkin's lymphoma (cHL) is chemotherapy and radiation therapy. However, some patients get relapsed, or their diseases become resistant. PD1 blocking antibodies have been used to increase the response of treatment in solid tumors, and led to potentially stable responses that are acceptable. Our purpose in this study is to investigate the effect of nivolumab as a PD1 blocking antibody on the survival rate of patients with Hodgkin's cancer.
Methods
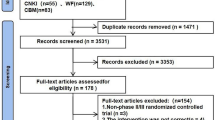
Databases were found in International Medical Sciences, Web of Science, Medline, Scopus, Index Copernicus, PubMed, DOAJ, Google Scholar, EBSCO-CINAHL, and Persian databases containing SID and Magiran using keywords such as: “checkpoint inhibitor”, “nivolumab”, “Hodgkin lymphoma”, and “PD1 Blockade”. The risk of bias was determined by two external observers using the Cochrane checklists. After the search, the data provided in 51 documents was independently evaluated. Duplicate papers were excluded. Assessing the full texts of the remaining papers, 7 papers were approved.
Results
Pooled data of these seven studies revealed that the overall objective response rate was 68% (CI 64.1% to 72.1%; heterogeneity; I2 = 40.19%; p = 0.123) with partial remission (52%; CI 46.5% to 57.6%; heterogeneity; I2 = 28.36%; p = 0.212). In the pooled analysis, complete remission was 16.8 (CI 11.1% to 26.4%). Pooled data of six studies showed that stable disease was averaged to 19% (CI 16% to 23%; heterogeneity; I2 = 30%; p = 0.209; fixed-effect model).
Conclusions
The results of the study indicate that nivolumab as a PD1 pathway inhibitor can be effective in treating relapsed and refractory cHL patients compared to other therapies, and lead to more effective treatment over the long term. Furthermore, the adverse effects of nivolumab are controllable and have a good safety profile.





Similar content being viewed by others
References
Makita S, Maruyama D, Maeshima AM, et al. Clinical features and outcomes of 139 Japanese patients with Hodgkin lymphoma. Int J Hematol. 2016;104(2):233–44.
Ogura M, Itoh K, Kinoshita T, et al. Phase II study of ABVd therapy for newly diagnosed clinical stage II–IV Hodgkin lymphoma: Japan Clinical Oncology Group study (JCOG 9305). Int J Hematol. 2010;92(5):713–24.
Keir ME, Butte MJ, Freeman GJ, et al. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704.
Weber J. Immune checkpoint proteins: a new therapeutic paradigm for cancer—preclinical background: CTLA-4 and PD-1 blockade. Semin Oncol. 2010;37(5):430–9.
Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 anti-body in patients with advanced cancer. N Engl J Med. 2012;366(26):2455–65.
Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–54.
Lipson EJ, Sharfman WH, Drake CG, et al. Durable cancer regression off-treatment and effective reinduction therapy with an anti-PD-1 antibody. Clin Cancer Res. 2013;19(2):462–8.
Taube JM, Klein AP, Brahmer JR, et al. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin Cancer Res. 2014;20(19):5064–74.
Andorsky DJ, Yamada RE, Said J, et al. Programmed death ligand 1 is expressed by non-hodgkin lymphomas and inhibits the activity of tumor-associated T cells. Clin Cancer Res. 2011;17(13):4232–44.
Armand P, Nagler A, Weller EA, et al. Disabling immune tolerance by programmed death-1 blockade with pidilizumab after autologous hematopoietic stem-cell transplantation for diffuse large B-cell lymphoma: results of an international phase II trial. J Clin Oncol. 2013;31(33):4199–206.
Berger R, Rotem-Yehudar R, Slama G, et al. Phase I safety and pharmacokinetic study of CT-011, a humanized antibody interacting with PD-1, in patients with advanced hematologic malignancies. Clin Cancer Res. 2008;14(10):3044–51.
Westin JR, Chu F, Zhang M, et al. Safety and activity of PD1 blockade by pidilizumab in combination with rituximab in patients with relapsed follicular lymphoma: a single group, open-label, phase 2 trial. Lancet Oncol. 2014;15(1):69–77.
Wilcox RA, Feldman AL, Wada DA, et al. B7-H1 (PD-L1, CD274) suppresses host immunity in T-cell lymphoproliferative disorders. Blood. 2009;114(10):2149–58.
Yang ZZ, Novak AJ, Stenson MJ, et al. Intratumoral CD4+CD25+ regulatory T-cell-mediated suppression of infiltrating CD4+ T cells in B-cell non-Hodgkin lymphoma. Blood. 2006;107(9):3639–46.
Keytruda (pembrolizumab). White-house Station, NJ: Merck (package insert). http://www.merck.com/product/usa/pi_circulars/k/keytruda/keytruda_pi.pdf.
Green MR, Monti S, Rodig SJ, et al. Integrative analysis reveals selective 9p24.1 amplification, increased PD-1 ligand expression, and further induction via JAK2 in nodular sclerosing Hodgkin lymphoma and primary mediastinal large B-cell lymphoma. Blood. 2010;116(17):3268–77.
Juszczynski P, Ouyang J, Monti S, et al. The AP1-dependent secretion of galectin-1 by Reed–Sternberg cells fosters immune privilege in classical Hodgkin lymphoma. Proc Natl Acad Sci USA. 2007;104(32):13134–9.
Steidl C, Shah SP, Woolcock BW, et al. MHC class II transactivator CIITA is a recurrent gene fusion partner in lymphoid cancers. Nature. 2011;471(7338):377–81.
Green MR, Rodig S, Juszczynski P, et al. Constitutive AP-1 activity and EBV infection induce PD-L1 in Hodgkin lymphomas and post transplant lymphoproliferative disorders: implications for targeted therapy. Clin Cancer Res. 2012;18(6):1611–8.
Kaufman D, Longo L. Hodgkin’s disease. In: Lenhord ER, Osteen R, Gansler T, editors. Clinical oncology. 2nd ed. Livingstone: Churchill; 2000. p. 2620–48.
Horning S. Hodgkin lymphoma. In: Beutler E, Lichtman M, Coller R, Kipps T, Seligsohn U, editors. Williams hematolog. 6th ed. New York: McGraw-Hill; 2001. p. 1215–28.
Fung HC, Nademanee AP. Approach to Hodgkin’s lymphoma in the new millennium. Hematol Oncol. 2002;20(1):1–15.
Ansell SM, Lesokhin AM, Borrello I, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N Engl J Med. 2015;372:311–9.
Abdi F, Roozbeh N. The effects of Humulus lupulus L.) hops) on menopausal vasomotor symptoms: a systematic review and meta-analysis. Iran J Obstetrics Gynecol Infer. 2016;19(26):9–17.
Maruyama D, Hatake K, Kinoshita T, et al. Multicenter phase II study of nivolumab in Japanese patients with relapsed or refractory classical Hodgkin lymphoma. Cancer Sci. 2017;108(5):1007–12.
Younes A, Santoro A, Shipp M, et al. Nivolumab for classical Hodgkin’s lymphoma after failure of both autologous stem-cell transplantation and brentuximab vedotin: a multicentre, multicohort, single-arm phase 2 trial. Lancet Oncol. 2016;17(9):1283–94.
Beköz H, Karadurmus N, Paydas S, et al. Nivolumab for relapsed or refractory Hodgkin lymphoma: real-life experience. Ann Oncol. 2017;28(10):2496–502.
Kasamon YL, de Claro RA, Wang Y, et al. FDA approval summary: nivolumab for the treatment of relapsed or progressive classical hodgkin lymphoma. Oncologist. 2017;22(5):585–91.
Armand P, Engert A, Younes A, et al. Nivolumab for relapsed/refractory classic Hodgkin lymphoma after failure of autologous hematopoietic cell transplantation: extended follow up of the multicohort single-arm phase II Check Mate 205 trial. J Clin Oncol. 2018;36(14):1428–39.
Herbaux C, Gauthier J, Brice P, et al. Efficacy and tolerability of nivolumab after allogeneic transplantation for relapsed Hodgkinlymphoma. Blood. 2017;129(18):2471–8.
Swerdlow SH, Campo E, Harris NL, et al. WHO classification of tumours of haematopoietic and lymphoid tissues. 4th ed. Geneva: World Health Organization; 2008.
Schmitz N, Pfistner B, Sextro M, et al. Aggressive conventional chemotherapy compared with high-dose chemotherapy with autologous haemopoietic stem-cell transplantation for relapsed chemosensitive Hodgkin’s disease: a randomised trial. Lancet. 2002;359(9323):2065–71.
Salihoglu A, Elverdi T, Karadogan I, et al. Brentuximab vedotin for relapsed or refractory Hodgkin lymphoma: experience in Turkey. Ann Hematol. 2015;94(3):415–20.
Younes A, Gopal AK, Smith SE, et al. Results of a pivotal phase II study of brentuximab vedotin for patients with relapsed or refractory Hodgkin’s lymphoma. J Clin Oncol. 2012;30(18):2183–9.
Cheah CY, Chihara D, Horowitz S, et al. Patients with classical Hodgkin lymphoma experiencing disease progression after treatment with brentuximab vedotin have poor outcomes. Ann Oncol. 2016;27(7):1317–23.
Martin-Liberal J, Furness AJ, Joshi K, et al. Anti-programmed cell death-1 therapy and insulin-dependent diabetes: a case report. Cancer Immunol Immunother. 2015;64(6):765–7.
Hughes J, Vudattu N, Sznol M, et al. Precipitation of autoimmune diabetes with anti-PD-1 immunotherapy. Diabetes Care. 2015;38(4):e55–7.
Okamoto M, Okamoto M, Gotoh K, et al. Fulminant type 1 diabetes mellitus with anti-programmed cell death-1 therapy. J Diabetes Investig. 2016;7(6):915–8.
Miyoshi Y, Ogawa O, Oyama Y. Nivolumab, an anti-programmed cell death-1 antibody, induces fulminant type 1 diabetes. Tohoku J Exp Med. 2016;239(2):155–8.
Acknowledgements
We would like to thank to Dr. Ramin Sadeghi from Mashhad University Department of Nuclear Medicine for his advice in the meta-analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval (research involving human participants and/or animals)
This work has no human or animal participants.
Informed consent
There is no consent for this work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Amraee, A., Evazi, M.R., Shakeri, M. et al. Efficacy of nivolumab as checkpoint inhibitor drug on survival rate of patients with relapsed/refractory classical Hodgkin lymphoma: a meta-analysis of prospective clinical study. Clin Transl Oncol 21, 1093–1103 (2019). https://doi.org/10.1007/s12094-018-02032-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-018-02032-4