Abstract
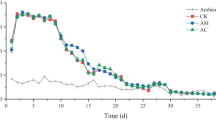
The effects of antibiotics on aerobic composting are investigated by dosing of tetracycline (TC) in fresh human feces with sawdust as biomass carrier. Variability in process parameters such as temperature, pH, water-soluble carbon, germination index (GI) and dehydrogenase activity (DHA) are evaluated at TC dosages of 0, 100, 250 and 500 mg/kg in a 21-day composting. Moreover, microbial community succession is examined by high-throughput 16S rRNA gene sequencing. Findings indicate significant impacts to the process parameters with the increase of TC concentration such as inhibition of temperature increases during aerobic composting, lowering of pH, increasing of water-soluble carbon residue, a decrease of GI, and hindering of DHA. Furthermore, elevated TC concentrations significantly alter the microbial community succession and reduce the community diversity and abundance. Therefore, interference in microbial community structures and a hindrance to biological activity are believed to be the main adverse effects of TC on the composting process and maturity of the composting products.







Similar content being viewed by others
References
Gao H, Zhou C, Li F, Han B, Li X (2015) Economic and environmental analysis of five Chinese rural toilet technologies based on the economic input–output life cycle assessment. J Clean Prod 8:91–98. https://doi.org/10.1016/j.jclepro.2015.12.089
Jenkins WM, Cumming O, Cairncross S (2015) Pit latrine emptying behavior and demand for sanitation services in Dar Es Salaam, Tanzania. Int J Environ Res Public Health 12:2588–2611. https://doi.org/10.3390/ijerph120302588
Bernal MP, Alburquerque JA, Moral R (2009) Composting of animal manures and chemical criteria for compost maturity assessment. A review. Bioresour Technol 100:5444–5453. https://doi.org/10.1016/j.biortech.2008.11.027
Kazama S, Tameike N, Nakagawa N, Otaki M (2011) A fate model of pathogenic viruses in a composting toilet based on coliphage inactivation. J Environ Sci 23:1194–1198. https://doi.org/10.1016/s1001-0742(10)60490-1
Lopez Zavala MA, Funamizu N, Takakuwa T (2004) Modeling of aerobic biodegradation of feces using sawdust as a matrix. Water Res 38:1327–1339. https://doi.org/10.1016/j.watres.2003.10.028
Du L, Liu W (2011) Occurrence, fate, and ecotoxicity of antibiotics in agro-ecosystems. A review. Agron Sustain Dev 32:309–327. https://doi.org/10.1007/s13593-011-0062-9
Heberer T (2002) Occurrence, fate, and removal of pharmaceutical residues in the aquatic environment: a review of recent research data. Toxicol Lett 131:5–17. https://doi.org/10.1016/S0378-4274(02)00041-3
Hirsch R, Ternes T, Haberer K, Kratz K-L (1999) Occurrence of antibiotics in the aquatic environment. Sci Total Environ 225:109–118. https://doi.org/10.1016/S0048-9697(98)00337-4
Björklund A(2002) The potential of using thermal composting for disinfection of separately collected faeces in Cuernacava, Mexico. Minor Field Studies No. 200. Swedish University of Agricultural Sciences, International Office, 1402–3237
Kakimoto T, Osawa T, Funamizu N (2007) Antibiotic effect of amoxicillin on the feces composting process and reactivation of bacteria by intermittent feeding of feces. Bioresour Technol 98:3555–3560. https://doi.org/10.1016/j.biortech.2006.11.029
Wu X, Wei Y, Zheng J, Zhao X, Zhong W (2011) The behavior of tetracyclines and their degradation products during swine manure composting. Bioresour Technol 102:5924–5931. https://doi.org/10.1016/j.biortech.2011.03.007
Eguchi K, Otawa K, Ohishi R, Nagase H, Ogata T, Nagai H, Murata N, Ishikawa H, Hirata K, Nakai Y (2012) Establishment of evaluation method to determine effects of veterinary medicinal products on manure fermentation using small-scale composting apparatus. J Biosci Bioeng 114:312–317. https://doi.org/10.1016/j.jbiosc.2012.04.001
Qian X, Sun W, Gu J, Wang XJ, Sun JJ, Yin YN, Duan ML (2016) Variable effects of oxytetracycline on antibiotic resistance gene abundance and the bacterial community during aerobic composting of cow manure. J Hazard Mater 315:61–69. https://doi.org/10.1016/j.jhazmat.2016.05.002
Arikan OA, Sikora LJ, Mulbry W, Khan SU, Foster GD (2007) Composting rapidly reduces levels of extractable oxytetracycline in manure from therapeutically treated beef calves. Bioresour Technol 98:169–176. https://doi.org/10.1016/j.biortech.2005.10.041
Sarmah AK, Meyer MT, Boxall AB (2006) A global perspective on the use, sales, exposure pathways, occurrence, fate and effects of veterinary antibiotics (VAs) in the environment. Chemosphere 65:725–759. https://doi.org/10.1016/j.chemosphere.2006.03.026
Hotta S, Funamizu N (2007) Biodegradability of fecal nitrogen in composting process. Bioresour Technol 98:3412–3414. https://doi.org/10.1016/j.biortech.2006.10.045
Li Q, Wang XC, Zhang HH, Shi HL, Hu T, Ngo HH (2013) Characteristics of nitrogen transformation and microbial community in an aerobic composting reactor under two typical temperatures. Bioresour Technol 137:270–277. https://doi.org/10.1016/j.biortech.2013.03.092
Wu S, Shen Z, Yang C, Zhou Y, Li X, Zeng G, Ai S, He H (2016) Effects of C/N ratio and bulking agent on speciation of Zn and Cu and enzymatic activity during pig manure composting. Int Biodeterior Biodegrad 119:429–436. https://doi.org/10.1016/j.ibiod.2016.09.016
Carman RJ, Simon MA, Petzold HE 3rd, Wimmer RF, Batra MR, Fernandez AH, Miller MA, Bartholomew M (2005) Antibiotics in the human food chain: establishing no effect levels of tetracycline, neomycin, and erythromycin using a chemostat model of the human colonic microflora. Regul Toxicol Pharmacol 43:168–180. https://doi.org/10.1016/j.yrtph.2005.06.005
Chantigny MH, Prévost D, Angers DA, Vézina L-P, Chalifour F-P (1996) Microbial biomass and N transformations in two soils cropped with annual and perennial species. Biol Fertil Soils 21:239–244. https://doi.org/10.1007/bf00334898
Gu W, Zhang F, Xu P, Tang S, Xie K, Huang X, Huang Q (2011) Effects of sulphur and Thiobacillus thioparus on cow manure aerobic composting. Bioresour Technol 102:6529–6535. https://doi.org/10.1016/j.biortech.2011.03.049
Feng L, Zhang L, Feng L (2014) Dissipation of polycyclic aromatic hydrocarbons in soil amended with sewage sludge compost. Int Biodeterior Biodegrad 95:200–207. https://doi.org/10.1016/j.ibiod.2014.04.012
Li C, Zhong S, Zhang F, Wang Z, Jiang F, Wan Y (2017) Response of microbial communities to supercritical CO2 and biogeochemical influences on microbially mediated CO2-saline-sandstone interactions. Chem Geol. https://doi.org/10.1016/j.chemgeo.2017.09.026
Lazarevic V, Gaïa N, Girard M, Schrenzel J (2016) Decontamination of 16S rRNA gene amplicon sequence datasets based on bacterial load assessment by qPCR. BMC Microbiol 16:1–8. https://doi.org/10.1186/s12866-016-0689-4
Wu X, Zhang H, Chen J, Shang S, Wei Q, Yan J, Tu X (2016) Comparison of the fecal microbiota of dholes high-throughput Illumina sequencing of the V3-V4 region of the 16S rRNA gene. Appl Microbiol Biotechnol 100:3577–3586. https://doi.org/10.1007/s00253-015-7257-y
Sun J, Qian X, Gu J, Wang X, Gao H (2016) Effects of oxytetracycline on the abundance and community structure of nitrogen-fixing bacteria during cattle manure composting. Bioresour Technol 216:801–807. https://doi.org/10.1016/j.biortech.2016.05.060
Diao XP, Sun YJ, Sun ZJ, Shen JZ (2006) Effects of three kinds of veterinary drugs on microbe respiration in different soils. J China Agric Univ 11:39–43
Wang X, Cui H, Shi J, Zhao X, Zhao Y, Wei Z (2015) Relationship between bacterial diversity and environmental parameters during composting of different raw materials. Bioresour Technol 198:395–402. https://doi.org/10.1016/j.biortech.2015.09.041
Liu B, Li Y, Zhang X, Feng C, Gao M, Shen Q (2015) Effects of composting process on the dissipation of extractable sulfonamides in swine manure. Bioresour Technol 175:284–290. https://doi.org/10.1016/j.biortech.2014.10.098
Bernai MP, Paredes C, Sánchez-Monedero MA, Cegarra J (1998) Maturity and stability parameters of composts prepared with a wide range of organic wastes. Bioresour Technol 63:91–99. https://doi.org/10.1016/S0960-8524(97)00084-9
Zucconi F, Pera A, Forte M, Bertoldi MD (1981) Evaluating toxicity of immature compost. Biocycle 22:54–57
Moharana PC, Biswas DR (2016) Assessment of maturity indices of rock phosphate enriched composts using variable crop residues. Bioresour Technol 222:1–13. https://doi.org/10.1016/j.biortech.2016.09.097
Vargas-García MC, Suárez-Estrella F, López MJ, Moreno J (2010) Microbial population dynamics and enzyme activities in composting processes with different starting materials. Waste Manag 30:771–778. https://doi.org/10.1016/j.wasman.2009.12.019
Barrena R, Vázquez F, Sánchez A (2008) Dehydrogenase activity as a method for monitoring the composting process. Bioresour Technol 99:905–908. https://doi.org/10.1016/j.biortech.2007.01.027
Wei X, Wu SC, Nie XP, Yediler A, Wong MH (2009) The effects of residual tetracycline on soil enzymatic activities and plant growth. J Environ Sci Health B 44:461–471. https://doi.org/10.1080/03601230902935139
Wong JWC, Fang M (2000) Effects of lime addition on sewage sludge composting process. Water Res 34:3691–3698. https://doi.org/10.1016/S0043-1354(00)00116-0
Xu S, Lu W, Liu Y, Ming Z, Liu Y, Meng R, Wang H (2017) Structure and diversity of bacterial communities in two large sanitary landfills in China as revealed by high-throughput sequencing (MiSeq). Waste Manag 63:41–48. https://doi.org/10.1016/j.wasman.2016.07.047
Zhang W, Huang MH, Qi FF, Sun PZ, Van Ginkel SW (2013) Effect of trace tetracycline concentrations on the structure of a microbial community and the development of tetracycline resistance genes in sequencing batch reactors. Bioresour Technol 150:9–14. https://doi.org/10.1016/j.biortech.2013.09.081
Takaku H, Kodaira S, Kimoto A, Nashimoto M, Takagi M (2006) Microbial communities in the garbage composting with rice hull as an amendment revealed by culture-dependent and -independent approaches. J Biosci Bioeng 101:42–50. https://doi.org/10.1263/jbb.101.42
Selvam A, Xu D, Zhao Z, Wong JW (2012) Fate of tetracycline, sulfonamide and fluoroquinolone resistance genes and the changes in bacterial diversity during composting of swine manure. Bioresour Technol 126:383–390. https://doi.org/10.1016/j.biortech.2012.03.045
Wang R, Zhang J, Sui Q, Wan H, Tong J, Chen M, Wei Y, Wei D (2016) Effect of red mud addition on tetracycline and copper resistance genes and microbial community during the full scale swine manure composting. Bioresour Technol 216:1049–1057. https://doi.org/10.1016/j.biortech.2016.06.012
Wang X, Pan S, Zhang Z, Lin X, Zhang Y, Chen S (2017) Effects of the feeding ratio of food waste on fed-batch aerobic composting and its microbial community. Bioresour Technol 224:397–404. https://doi.org/10.1016/j.biortech.2016.11.076
de Gannes V, Eudoxie G, Hickey WJ (2013) Prokaryotic successions and diversity in composts as revealed by 454-pyrosequencing. Bioresour Technol 133:573–580. https://doi.org/10.1016/j.biortech.2013.01.138
Cucurachi M, Busconi M, Marudelli M, Soffritti G, Fogher C (2013) Direct amplification of new cellulase genes from woodland soil purified DNA. Mol Biol Rep 40:4317–4325. https://doi.org/10.1007/s11033-013-2519-1
Domínguez-Mendoza CA, Bello-López JM, Navarro-Noya YE, de León-Lorenzana AS, Delgado-Balbuena L, Gómez-Acata S, Ruíz-Valdiviezo VM, Ramirez-Villanueva DA, Luna-Guido M, Dendooven L (2014) Bacterial community structure in fumigated soil. Soil Biol Biochem 73:122–129. https://doi.org/10.1016/j.soilbio.2014.02.012
Salinas-Martínez A, de los Santos-Córdova M, Soto-Cruz O, Delgado E, Pérez-Andrade H, Háuad-Marroquín LA, Medrano-Roldán H (2008) Development of a bioremediation process by biostimulation of native microbial consortium through the heap leaching technique. J Environ Manag 88:115–119. https://doi.org/10.1016/j.jenvman.2007.01.038
Stolz A, Burger S, Kuhm A, Kampfer P, Busse HJ (2005) Pusillimonas noertemannii gen. nov., sp. nov., a new member of the family Alcaligenaceae that degrades substituted salicylates. Int J Syst Evol Microbiol 55:1077–1081. https://doi.org/10.1099/ijs.0.63466-0
Ligi T, Oopkaup K, Truu M, Preem J-K, Nõlvak H, Mitsch WJ, Mander Ü, Truu J (2014) Characterization of bacterial communities in soil and sediment of a created riverine wetland complex using high-throughput 16S rRNA amplicon sequencing. Ecol Eng 72:56–66. https://doi.org/10.1016/j.ecoleng.2013.09.007
Acknowledgements
This work was supported by National Natural Science Foundation of China (Grant No. 2010116062) and the China Postdoctoral Science Foundation (Grant No. 2016M602781).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Shi, H., Wang, X.C., Li, Q. et al. Effects of Elevated Tetracycline Concentrations on Aerobic Composting of Human Feces: Composting Behavior and Microbial Community Succession. Indian J Microbiol 58, 423–432 (2018). https://doi.org/10.1007/s12088-018-0729-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12088-018-0729-x