Abstract
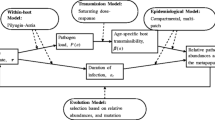
There is ongoing interest in the conditions that favor the evolution of acute, highly transmissible infections in contrast to chronic ones. Earlier studies typically consider the evolution of a trait that is constant over the lifetime of an infection. However, for many pathogens, such traits can vary over this course. Here, we address the evolution of temporal patterns in limited host population sizes, where a trade-off between invasion and persistence can arise. This is of particular relevance to questions on the evolution of acuteness and chronicity. We ask whether population dynamics of transmission at the between-host level could lead pathogen adaptation to favor temporal strategies during the course of infection. To do this, we consider an infection to be composed of multiple stages, allowing each of these to evolve independently under a transmission–duration trade-off. We only consider selection taking place on the between-host level and examine the balance of invasion and persistence (i.e., maximizing replication vs. minimizing vulnerability to extinction), using several fitness-related measures. We find that a composite strategy that is ordered in time can confer higher fitness than any single, constant, strategy. We discuss the relevance of these results for the ordered expression of var genes in Plasmodium falciparum, as well as for infections that characteristically have several stages as in some bacterial pathogens.





Similar content being viewed by others
References
Alizon S (2008) Transmission–recovery trade-offs to study parasite evolution. Am Nat 172:E113–E121
Alizon S et al (2009) Virulence evolution and the trade-off hypothesis: history, current state of affairs and the future. J Evol Biol 22:245–259
Anderson H, Britton T (2000) Stochastic epidemics in dynamic populations: quasi-stationarity and extinction. J Math Biol 41:559–580
Anderson RM, May RM (1979) Population biology of infectious diseases. Nature 280:361–367
Anderson H, May RM (1981) The population-dynamics of micro-parasites and their invertebrate hosts. Philos Trans R Soc Lond 1981(291):451–524
Anderson RM, May RM (1982) Coevolution of hosts and parasites. Parasitology 85:411–426
Artur Scherf A et al (2008) Antigenic variation in Plasmodium falciparum. Annu Rev Microbiol 62:445–470
Bartlett MS (1956) Measles periodicity and community size. J R Stat Soc 120:48–60
Bartlett MS (1957) Measles periodicity and community size. J R Stat Soc Ser A 1957:48–60
Bjørnstad ON, Harvill ET (2005) Evolution and emergence of Bordetella in humans. Trends Microbiol 13(8):355–359
Boerlijst MC, van Ballegooijen WM (2010) Spatial pattern switching enables cyclic evolution in spatial epidemics. PLoS Comput Biol 6(12):e1001030
Bremermann HJ, Pickering J (1983) A game-theoretical model of parasite virulence. J Theor Biol 100:411–426
Bull JJ (1994) Virulence. Evolution 48:1423–1437
Bull PC et al (2000) Plasmodium falciparum-infected erythrocytes: agglutination by diverse Kenyan plasma is associated with severe disease and young host age. J Infect Dis 182:252–259
Bull PC et al (2005) Plasmodium falciparum variant surface antigen expression patterns during malaria. PLoS Pathog 1(3)
CDC (2011) Epidemiology and prevention of vaccine-preventable diseases, The Pink Book: Course Textbook. CDC, Atlanta
Day T (2001) Parasite transmission modes and the evolution of virulence. Evolution 55:2389–2400
Day T (2003) Virulence evolution and the timing of disease life-history events. Trends Ecol Evol 18:113–118
Day T et al (2011) Bridging scales in the evolution of infectious disease life histories: theory. Evolution 65(12):3448–3461. doi:10.1111/j.1558-5646.2011.01394.x
Dietz K (1975) Transmission and control of arbovirus diseases. In: Ludwig D, Cooke KL (eds) Epidemiology. Society for the Industrial and Applied Mathematics, Philadelphia
Ebert D, Herre EA (1996) The evolution of parasitic diseases. Parasitol Today 12:96–100
Ewald PW (1983) Host–parasite relations, vectors, and the evolution of disease severity. Annu Rev Ecol Evol Syst 14:465–485
Frank SA (1996) Models of parasite virulence. Q Rev Biol 71:37–38
Gardner MJ et al (2002) Genome sequence of the human malaria parasite Plasmodium falciparum. Nature 419:498–511
Gillespie DT (1977) Exact stochastic simulation of coupled chemical reactions. J Phys Chem 81:2340–2361
Grenfell BT (2001) Dynamics and epidemiological impact of microparasites. In: Irving WL, Smith G, McCauley JW, Rowlands DJ (eds) New challenges to health: the threat of virus infection. Cambridge University Press, Cambridge, pp 33–52
Kaestli M et al (2006) Virulence of malaria is associated with differential expression of Plasmodium falciparum var gene subgroups in a case-control study. J Infect Dis 193:1567–1574
Keeling MJ, Grenfell BT (1997) Disease extinction and community size: modeling the persistence of measles. Science 275:65–67
Keeling MJ, Rohani P (2008) Modeling infectious diseases in humans and animals. Princeton University Press, Princeton
King AA et al (2009) Evolution of acute infections and the invasion–persistence trade-off. Am Nat 173:446–455
Lenski RE, May RM (1994) The evolution of virulence in parasites and pathogens: reconciliation between two competing hypotheses. J Theor Biol 169:253–265
Lipsitch M et al (1995) The population dynamics of vertically and horizontally transmitted parasites. Proc Roy Soc B 260:321–327
Massad E (1987) Transmission rates and the evolution of pathogenicity. Evolution 41:1127–1130
Mideo N et al (2008) Linking within- and between-host dynamics in the evolutionary epidemiology of infectious diseases. Trends Ecol Evol 23:511–517
Mideo N et al (2011) Bridging scales in the evolution of infectious disease life histories: application. Evolution 65:3298–3310. doi:10.1111/j.1558-5646.2011.01382.x
Miller LH et al (2002) The pathogenic basis of malaria. Nature 415:673–679
Nasell I (1999) On the time to extinction in recurrent epidemics. J R Stat Soc Ser B 61:309–330
Nasell I (2005) A new look at the critical community size for childhood infections. Theor Popul Biol 67:203–216
Peters J et al (2002) High diversity and rapid changeover of expressed var genes during the acute phase of Plasmodium falciparum infections in human volunteers. PNAS 99(16):10689–10694
Read AF (1994) The evolution of virulence. Trends Microbiol 2:73–76
Recker M et al (2004) Transient cross-reactive immune responses can orchestrate antigenic variation in malaria. Nature 429:555–558
Recker M et al (2011) Antigenic variation in Plasmodium falciparum malaria involves a highly structured switching pattern. PLoS Pathog 7:e1001306
Sasaki A, Iwasa Y (1991) Optimal growth schedule of pathogens within a host: switching between lytic ad latent cycles. Theor Popul Biol 39:201–239
Severins M et al (2012) How selection forces dictate the variant surface antigens used by malaria parasites. J R Soc Interface 9:246–260
Taylor PD et al (2006) The evolutionary consequences of plasticity in host–pathogen interactions. Theor Popul Biol 69:323–331
van Noort SP et al (2010) Immune selection and within-host competition can structure the repertoire of variant surface antigens in Plasmodium falciparum—a mathematical model. PLoS ONE 5:e9778
Wallinga J, Lipsitch M (2007) How generation intervals shape the relationship between growth rates and reproductive numbers. Proc R Soc B Biol Sci 274(1609):599–604. doi:10.1098/rspb.2006.3754
Warimwe GM et al (2009) Plasmodium falciparum var gene expression is modified by host immunity. PNAS 106(51):21801–21806. doi:10.1073/pnas.0907590106
Weiss RA (2002) Virulence and pathogenesis. Trends Microbiol 10(7):314–318
Acknowledgments
MP is a Howard Hughes Medical Investigator.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 2.95 mb)
Rights and permissions
About this article
Cite this article
Artzy-Randrup, Y., Pascual, M. Composite temporal strategies in pathogen evolution: balancing invasion and persistence. Theor Ecol 7, 325–334 (2014). https://doi.org/10.1007/s12080-014-0221-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12080-014-0221-0